Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nanomedicines
Tiny Tech To Treat Cancer
Sophisticated therapeutics based on nanotechnology make their way to the clinic
by Bethany Halford
June 4, 2012
| A version of this story appeared in
Volume 90, Issue 23

When fighting an enemy as deadly and resilient as cancer, doctors use powerful medical weaponry: Excise the tumors. Blast them with radiation. Choke them with chemotherapy. When physicians take this scorched-earth approach, as they often have to, they hope they can kill the cancer without also killing their patients.
“Patients at times will decline therapies because undergoing them is so painful,” says Piotr Grodzinski, a director of the National Cancer Institute’s Alliance for Nanotechnology in Cancer. “Chemotherapies used right now are applied systemically—they impact diseased tissue as well as healthy tissue,” he explains. That could change by bringing a therapy directly to the cancer.
A new generation of nanoparticle-based therapies aims to do just that. By virtue of their small size and, in some cases, their chemical composition, these nanoscale therapeutics can slip into tumors and even individual cancer cells while steering clear of healthy tissue.
Scientists have been hoping to take advantage of nanotechnology in this manner for more than a decade, and now patients are finally beginning to see the fruits of that labor as sophisticated nanoparticle therapeutics make their way into clinical trials.
Simple nanoparticle-based drugs are already on the market. Many point to Doxil as the first example of a nanotechnology-enabled therapeutic. It was approved in 1995 for the treatment of AIDS-related Kaposi’s sarcoma and later for ovarian cancer. In Doxil, the small-molecule therapeutic doxorubicin is trapped within a nanoscale liposome—a sort of bubble made from a lipid bilayer.
Doxil also has a layer of methoxypolyethylene glycol strands, which help the liposome evade the body’s immune system. The drug thus stays in the body longer, giving it more time to reach tumor-riddled tissue.
Similarly, Abraxane uses albumin-based nanoparticles to deliver the drug paclitaxel. It was approved in 2005 to treat breast cancer, and its use has since been expanded for other types of cancer. The albumin particles make it possible to give higher doses of the taxane, which is sparingly soluble in blood.
But these formulations aren’t nanotechnology at its best, says Mark E. Davis, a chemical engineering professor at California Institute of Technology who has been working to develop cancer-fighting nanotherapeutics for more than 15 years. Their mechanism of action doesn’t take advantage of what working at the nanoscale offers, specifically the ability to slip into tumors and cancerous cells and deliver a large payload of drug there.
The body interacts with nanoscale objects differently than it does with small molecules, Davis explains. Chemotherapy drugs, he says, can travel all over the body and wreak havoc on systems that aren’t even affected by cancer.
“You can stop many of these unwanted interactions by just creating something that’s nanoscale,” he notes. “That length scale allows you to significantly alter these drugs to keep them from going where you don’t want them to go and to effectively get more of them to tumors. It allows you to do things you can’t do with small-molecule chemo drugs,” Davis says.

The blood vessels that run through tumors are poorly formed, and consequently they are full of leaks. Nanoparticles less than 100 nm or so in diameter easily slip into these leaks and get stuck via what’s known as the enhanced permeability and retention, or EPR, effect.
In addition, “newer nanoparticles have very high function relative to basic liposomes,” Davis adds. They can be tailored to control drug release rates, for example, or they can carry targeting ligands that make the nanoparticles interact more strongly with cancer cells.
Davis’ own work developing nanoscale therapeutics got started in the mid-1990s, after his wife, Mary, went through a grueling round of chemotherapy to treat breast cancer. “She basically said, ‘Is there a better way to treat patients? Because this is just awful,’ ” he recalls. At the time, Davis’ research was in heterogeneous catalysis—far afield from treating cancer. But Mary convinced him it was worth a shot, so at age 40, with no medical training, Davis entered the world of nanomedicine.
In principle, Davis says, the concept is simple: Make a nanoparticle and somehow load it with a cancer-killing agent that will be strategically released once it reaches a tumor. In practice, however, creating a particle that will remain an individual particle, that will circulate, and that will not stick where it’s not supposed to stick was a real challenge.
Now, one of the technologies invented in Davis’ lab has reached Phase II clinical trials to treat patients with advanced non-small-cell lung cancer. The treatment, known as CRLX101, has been licensed by Cambridge, Mass.-based Cerulean Pharma.
CRLX101 features the small-molecule chemotherapeutic camptothecin covalently connected to a β-cyclodextrin-polyethylene glycol copolymer via a glycine linker. Under the right conditions, camptothecin moieties on some polymer strands will slip into the hydrophobic cores of the cyclodextrin groups on other strands. These polymer tangles take the shape of nanoparticles 20–50 nm in diameter.
The camptothecin is conjugated via an ester bond to the glycine linker and the copolymer backbone, explains Sonke Svenson, Cerulean’s director of research. This structure is stable as it circulates in the blood at around pH 7. When it encounters the acidic environment of tumor cells, which are roughly pH 5 or 6, the ester bond hydrolyzes, releasing free camptothecin. The leftover strands of polymer are then excreted through the renal system.
Camptothecin, it turns out, failed as a small-molecule chemotherapeutic agent decades ago. It’s a good inhibitor of the cancer target topoisomerase I, but the molecule suffers from poor solubility in blood and causes adverse drug reactions. Also, its structure features a lactone ring, which easily opens up, deactivating the drug.
“By conjugating camptothecin to a polymer nanoparticle, the drug gets delivered inside tumor cells. It’s right where you want it to be,” explains Scott Eliasof, Cerulean’s vice president of research. “It’s a very potent drug, it just needed a delivery system.”
To date, more than 150 patients have been treated with CRLX101 with acceptable side effects. Results indicate that the drug increases progression-free survival times in advanced lung cancer.

What’s more, Svenson says, side effects are minimal compared with other treatments, including highly tolerated small-molecule inhibitors like erlotinib and imatinib. “It’s not like a typical cancer treatment, where you suffer from cancer, you suffer from the treatment, then you suffer from cancer again,” he says. “People can actually maintain a good quality of life during the treatment.”
Researchers at Bind Biosciences say they’re also seeing few side effects with their polymer-based nanoparticle chemotherapeutic, BIND-014. The drug, which is in Phase I clinical trials, is built on the company’s Accurin platform.
Accurin nanoparticles are made up of a copolymer composed of a hydrophobic polyester section and a hydrophilic polyethylene glycol section. In water, strands of these copolymers form nanoparticles with the hydrophilic section on the surface and the hydrophobic section at the core, where a variety of chemotherapeutic agents can be trapped.
Accurins also feature targeting ligands, which are biological molecules that interact with proteins on cancer cells and cause the particles to associate more strongly on those cells. These ligands are covalently attached to the end of the hydrophilic portion on some strands of the copolymer, decorating the particle’s surface.
The modular nature of the particles makes it easy to build in different properties, says Jeff Hrkach, Bind’s senior vice president of technology R&D. “By virtue of the components we use and the processes we’ve developed, we’re able to have programmability. We program in the drug we want to use at the appropriate load and at the appropriate release profile. We can program in the size and the surface charge of the nanoparticle, as well as which targeting ligand we want to use.”
For BIND-014, the targeting ligand is a small molecule that binds to prostate-specific membrane antigen, a transmembrane protein found on the surface of prostate cancer cells and on the blood vessels of nonprostate solid tumors. BIND-014 carries the chemotherapeutic docetaxel.

“When you think of it, this is similar to the way pharma guys develop drugs,” says Omid C. Farokhzad, cofounder of Bind Biosciences and a professor at Harvard Medical School. Farokhzad invented this type of targeted polymeric nanoparticle with Robert S. Langer when he was a postdoc in Langer’s lab at Massachusetts Institute of Technology a decade ago. “Medicinal chemists start off with libraries of compounds that narrowly vary from one another chemically and then screen them to come up with the perfect molecule.” With Accurins, scientists can similarly adjust the nanoparticle to give it specific pharmacological properties.
For example, thanks to the Accurin structure, the pharmacology of BIND-014 is nothing like that of docetaxel, Farokhzad says. “It’s a fundamentally different cancer drug that has the potential to make a very big dent in oncology.”
At the moment, Bind is conducting Phase I clinical trials to establish the maximum tolerated dose of BIND-014. The small study has seen some promising results, with some patients’ tumors responding to BIND-014 even though they didn’t respond to docetaxel on its own.
Nanoparticles aren’t limited to delivering small-molecule chemotherapeutics. The technology is also well suited for carrying delicate biomolecular cargo, such as silencing RNA (siRNA), which would otherwise be destroyed quickly by the body (C&EN, Sept. 7, 2009, page 18).
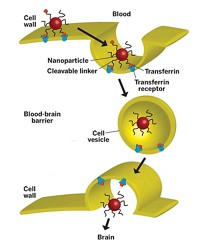
Calando Pharmaceuticals, a company that uses the polymer nanoparticles developed in Davis’ lab at Caltech, has one such product in Phase I clinical trials for treating solid tumors. The drug, known as CALAA-01, is part of Calando’s RONDEL platform.
RONDEL nanoparticles feature linear polymers containing positively charged groups alternating with cyclodextrin moieties. When mixed with siRNA, the negatively charged nucleic acid and the positively charged polymer come together to form nanoparticles less than 100 nm in diameter, with the siRNA at their cores and cyclodextrin groups on their surfaces.
To add targeting ligands to the polymer surface, such biomolecules are attached to polyethylene glycol strands that have adamantane groups at the opposite end. These adamantanes act like anchors as they slip within the cyclodextrin groups, leaving the polymer and targeting ligands dangling from the particle.
CALAA-01 uses transferrin, a blood plasma protein known to associate with the receptors of cancer cells, as its targeting ligand. When CALAA-01 reaches its target cancer cells, the nanoparticle gets taken into the cell via endocytosis. Once there, the nanoparticle unfurls, releasing the siRNA into the cytoplasm, where it interacts with the cell’s machinery and shuts the cancer cell down.
Alnylam Pharmaceuticals also has a nanoparticle-based therapeutic for delivering siRNA. The therapy, known as ALN-VSP, has just completed Phase I clinical trials to treat liver cancer. ALN-VSP packages its siRNA within a nanoscale liposome first developed by Tekmira Pharmaceuticals.
“With liposomal nanoparticles, we surround the siRNA in a lipid barrier that’s shaped, sized, and charged to effectively deliver it into the liver, liver hepatocytes, and liver cancer cells,” explains Barry E. Greene, Alnylam’s president and chief operating officer.
“In the case of RNA interference, nanotechnology has allowed us to effectively and safely move into the clinic and achieve clinical results with RNAi therapeutics that we have never seen before,” Greene notes. Alnylam is hoping to partner with a larger company to take ALN-VSP into Phase II clinical trials.
Not all nanoparticles rely on attached or encapsulated molecules to fight tumors. Some do the cancer-killing work themselves.

Nanobiotix, for example, recently began clinical trials in France of its NanoXray system, which uses hafnium oxide nanocrystals along with standard radiation treatment to destroy cancer cells.
“Radiotherapy is one of the key technologies used in the treatment of cancer,” says Laurent Lévy, Nanobiotix’ chief executive officer. During radiation therapy, the body is exposed to X-rays, which prompt water molecules in the body to form free radicals that destroy a cell’s DNA. “You can kill any type of cancer cell using radiotherapy. It is just a matter of dose.”
Advertisement
The problem, Lévy explains, is that to reach the tumor, X-rays must also cross healthy tissue, potentially causing damage there as well. The key need for radiotherapy, he says, is to increase the dose of radiation within the tumor without increasing the dose within the healthy tissue.
Nanobiotix achieves selective dosing with HfO2 nanocrystals. Although the company is working on several different delivery systems, the one in trials uses crystals 50 nm in diameter, called NBTXR3. These crystals are injected directly into tumors, where they then slip into cancer cells.
Thanks to hafnium’s high electron density, these crystals create more radicals than water alone when exposed to X-rays, effectively upping the radiation dose at the tumor site. They’re otherwise inert and get cleared from the body in a matter of months, allowing for several rounds of radiation treatment.
“All we add to the existing standard of care is one injection,” Lévy points out. “The potential extra cost to the health care system is not that high.”
Because the therapy takes advantage of a physical effect, rather than a chemical or biological one, the technology is regulated as a device rather than as a drug.
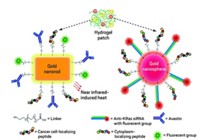
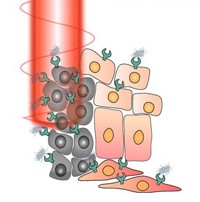
That’s also the case with a nanoparticle-based therapy that Nanospectra Biosciences has in clinical trials. The technology, known as AuroLase Therapy, was invented by Rice University scientists Naomi J. Halas and Jennifer L. West.
Patients get a systemic infusion of gold nanoshells roughly 150 nm in diameter, known as AuroShells. These particles slip into tumors and get caught there, thanks to the EPR effect. The particles are given 12 to 24 hours to accumulate within tumors or else be removed from the bloodstream. Then patients undergo treatment with a near-infrared laser. The near-IR light easily penetrates tissue and heats the nanoshells, which then destroy the tumors in which they’ve been trapped. Healthy tissue around the tumor remains unscathed.
“Because the treatment is thermal, there are no tumors or cancers that are resistant,” explains Glenn P. Goodrich, a vice president at Nanospectra. “If you can heat the cells high enough, they’re going to die.”
The technology allows treatment of tumors that might be difficult to remove via surgery, such as those wrapped around arteries or nerve bundles. “Because our treatment doesn’t harm healthy tissue, we should be able to treat right up to those critical areas,” Goodrich says. He also notes that because the particles themselves are inert, there’s no toxicity involved with the treatment.
Nanospectra currently has two clinical trials going with AuroLase: one in the U.S. to treat tumors of the head and neck and one in Mexico to treat prostate tumors.
“In this type of health care environment, you’ve got to show you’re better than what’s out there,” says John K. Stroh, Nanospectra’s president and CEO. “Nanoparticles have been around for quite a while. There’s still a little bit of mystique about them, which makes them novel in some ways but unfamiliar in other ways.”
“Demonstrating to doctors that we can provide something that is an attractive and perhaps better treatment for their patients than the more traditional techniques is a big challenge that everybody in nanotechnology faces,” Goodrich adds.
“This field is still really just on the cusp of getting into the clinic,” remarks Caltech’s Davis. “What we need for the field is to see some good Phase II and Phase III clinical trial data to get the medical community really interested. Phase I trials are great, but for the medical community, you really need to get out into these later trials where you’re treating lots of patients and getting statistically meaningful data.”
“Oncologists really do want breakthrough agents that result in clinically meaningful improvements in survival for patients with advanced cancer and, even better, that can be used to cure patients with cancer,” says Roger B. Cohen, a professor of medicine at the University of Pennsylvania, the associate director for clinical research at Penn’s Abramson Cancer Center, and a member of Cerulean Pharma’s medical advisory board. “Certainly nanomedicines offer that promise,” he says.
Whether nanoscale therapeutics will deliver on that promise remains to be seen, Cohen says. “We’re about to deal with a whole new generation of nanomedicines, and we do need to proceed with our usual deliberation and monitor patients for unwanted toxicities that could undermine the entire field if we’re not careful,” he says. All the companies C&EN spoke with say their products have been through extensive safety testing.
“We have a number of companies working in nanomedicines,” Cohen notes. “At this particular moment each has a unique platform—platforms that are largely untested in patients. Just looking at the landscape broadly as an oncologist and as a Phase I investigator, I don’t think any of us can say that one of these companies’ technologies is better than any other. I think there is significant enthusiasm for all of them.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter