Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
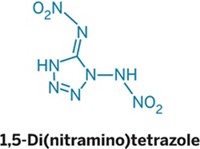
Chock Full of Nitrogen
New syntheses could lead to a solid all-nitrogen compound that's stable under ambient conditions
by Stephen K. Ritter
October 11, 2004
| A version of this story appeared in
Volume 82, Issue 41
A solid all-nitrogen compound at room temperature and pressure? That's an intriguing thought. Even better would be an all-nitrogen compound that's stable enough to be handled and processed into useful materials. The possibility that such a compound might be made is getting closer to fruition, as evidenced by several papers in the Sept. 20 issue of Angewandte Chemie International Edition.
On page 4919 of that issue, Ralf Haiges, Karl O. Christe, and coworkers at the University of Southern California report the preparation and spectroscopic characterization of new pentanitrogen cation salts, including N5+P(N3)6- and N5+B(N3)4-. On page 4924, My-Hang V. Huynh and Michael A. Hiskey of Los Alamos National Laboratory and coworkers report the synthesis and full characterization of an azobis(diazidotriazine) (C6N20). And on page 4834, Carsten Knapp and Jack Passmore of the University of New Brunswick, Fredericton, provide a commentary on the progress being made in the synthesis of high-nitrogen-content compounds and hint at the possibility of "solid nitrogen."
Several solid nitrogen-rich compounds reported in the past few years have come remarkably close to 100% nitrogen, in some cases being only one atom away. These compounds, besides being an interesting gambit to gain fundamental chemical knowledge, are potential candidates as commercial high-energy-density materials (HEDMs) used as explosives, propellants, and fireworks.
Earlier this year, a single-bonded nitrogen allotrope, called polymeric nitrogen, was made by compressing N2 above 110 gigapascals (about 1.1 million atm) and 2,000 K (C&EN, Aug. 2, page 10). This all-nitrogen compound has a diamond-like structure and is stable at room temperature at pressures above 42 GPa, but it doesn't seem likely that it can be isolated at ambient pressure without stabilizing it with other elements. Thus, preparing high-nitrogen-content compounds by traditional synthetic chemistry appears to still have the inside track.
Christe's group first prepared the N5+ cation and isolated it as the "marginally stable" AsF6- salt in 1999. Christe and coworkers later made the more stable SbF6- salt and have tried to make the all-nitrogen compound N5+N3-. But even the usually stable azide anion (N3-) has not allowed their attempts to end in anything other than explosions. They also have identified the cyclic N5- anion in the gas phase, which opened another avenue to explore, including the possibility of preparing N5+N5-. Yet, the researchers are giving up on trying to make these all-nitrogen compounds because theoretical calculations indicate that they simply are too unstable to isolate as solids.
Haiges and Christe report that they have come pretty close to an all-nitrogen molecule anyway with their latest N5+ salts. The extremely shock-sensitive N5P(N3)6 and N5B(N3)4 were prepared by reacting N5SbF6 with NaP(N3)6 or NaB(N3)4 in liquid SO2 at -64 °C. The boron compound, with a molecular formula of BN17, sets a new record for the most nitrogen content in a solid: 95.7 weight %, Christe says.
Hiskey and his collaborators have been taking a different approach for preparing HEDMs. For several years, the Los Alamos researchers have been synthesizing a variety of compounds containing tetrazine (C2N4) rings with nitrogen substituent groups. A natural extension of the work has been to make the azotriazines, Hiskey says, and the team realized that azido-substituted azobis(triazines) would have a very high positive heat of formation, giving them great explosive power. Huynh, Hiskey, and coworkers then synthesized the azobis(diazidotriazine) in three steps from a hydrazobis(dichlorotriazine).
Traditional polynitro compounds, such as trinitrotoluene (TNT), derive their explosive power from combustion of the carbon backbone of the molecule using the oxygen carried by the nitro groups, they note. Newer polynitro compounds gain additional power by having higher nitrogen content coupled with the strain of small rings or cage structures. The latest nitrogen-rich compounds--tetrazines and triazines--can unleash even greater force with their large number of N–N bonds, Hiskey and coworkers add. As a bonus, these compounds primarily form N2 on detonation, an environmentally friendlier by-product than those from traditional HEDMs.
FOR COMPARISON, TNT has a heat of formation of -64 kJ per mol, whereas 2,4,6-tri(azido)-1,3,5-triazine (cyanuric azide) has a positive heat of formation of 1,053 kJ per mol. Huynh and Hiskey's triazine compound far surpasses that level at 2,171 kJ per mol. Its energy density of 6.17 kJ per g is close to the value of 6.84 kJ per g estimated for N5P(N3)6.
Knapp and Passmore's commentary summarizes recent work on tellurium and other polyazido compounds synthesized by Christe's group and a group led by Thomas M. Klapötke of the University of Munich, in Germany. Last year, both groups independently reported the synthesis of Te(N3)4 as well as compounds containing Te(N3)5-, and Christe's group made a Te(N3)62- compound. Also, earlier this year Christe's group reported the analogous titanium polyazides. Passmore's group carried out some of the early work on the tellurium azides in the late 1980s. The sulfur and selenium homologs are still unknown, Knapp and Passmore point out.
Klapötke's group has prepared and studied a number of HEDMs, including many main-group azides in addition to the tellurium compounds. Christe's N5+ phosphorus and boron azido salts may be too sensitive to be used as HEDMs, Klapötke notes. But the triazine made by Hiskey's group "may well be of interest as an HEDM in suitable formulations," he adds.
"These developments show that some of the most outrageous compounds imaginable can be prepared and characterized," Knapp and Passmore conclude. They believe many other simple species with even greater nitrogen content will be isolated and characterized in the next few years--perhaps even an all-nitrogen compound.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter