Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Harnessing Microreactions
Researchers find that processes run in microreactors open doors to more efficient and novel chemistry useful for fine chemicals and intermediates
by Ann M. Thayer, C&EN Houston
May 30, 2005
| A version of this story appeared in
Volume 83, Issue 22

Xi'an Huian Chemical, with the help of collaborators from Germany's Institute for Microtechnology Mainz (IMM), has started producing nitroglycerin in the range of 10 kg per hour at a plant in the middle of China. The poisonous, explosive compound is destined for medical use. And the highly exothermic, potentially dangerous production process that uses extremely acidic reactants runs efficiently and safely, says Volker Hessel, IMM's vice scientific director.
The key to this production process is microreactor technology, which employs a system of often miniaturized reactors, mixers, heat exchangers, and other processing elements with internal structures on a micrometer scale (10 to 500 µm). Continuous processing based on flow chemistry alone is one advantage. But because of the small channel sizes and high surface area-to-volume ratios, these devices are orders of magnitude more efficient than large-scale batch reactors in heat and mass transfer.
Better heat and mass transfer, in turn, can contribute to improved conversion, selectivity, yield, safety, and product quality. Improved heat transfer allows reactions to be run at room temperature, for example, instead of under costly cryogenic conditions used to subdue reactions. Rapid and effective mixing brings reactants into contact for better conversion. Fast reactions, along with controlled residence times and temperatures, can generate the desired product without unwanted by-products or side reactions, resulting in higher selectivity and yield.
Numerous reactions, including many notable and industrially relevant reactions, have been tried out successfully in microreactors. Indeed, more than 400 publications detailing these successes have appeared in peer-reviewed journals, according to Hessel. Extensive reviews by him and his colleagues (Curr. Org. Chem. 2005, 9, 765; Chem. Eng. Technol. 2005, 28, 267) and by University of Hull chemists Paul Watts and Stephen J. Haswell (Chem. Eng. Technol. 2005, 28, 290) cover the wealth of reactions tested.
It's not surprising then that researchers in industry, academia, and at microtechnology R&D centers believe that the technology is being tested widely at the R&D level. "All the huge companies involved in chemistry and pharmaceutical chemistry are doing something in microreaction technology," surmises Stefan Löbbecke, vice director of the energetic materials department at Fraunhofer Institute for Chemical Technology (ICT) in Pfinztal, Germany.
Although the details of the work under way are somewhat scarce, "the direction is now more and more into the pilot and production scale," Hessel comments. Evidence of this is found, for example, in the patent literature, which, according to Hessel, "shows an increasing number of references on industrial microreactor-based chemical processes." Industrial participants also are frequenting more conferences and meetings.
Microreactor research has accelerated during the past 10 to 15 years. IMM and Fraunhofer ICT are among several R&D organizations worldwide that are developing, using, and promoting the technology. Six Fraunhofer institutes across Germany have joined together to create the Fraunhofer Alliance for Modular Microreaction Systems (FAMOS). Other organizations include the New Jersey Center for MicroChemical Systems (NJCMCS) at Stevens Institute of Technology and the MicroChemical Process Technology Research Association (MCPT) in Japan.
Along with institutes offering systems to their collaborators and customers, commercial suppliers of microreactors for research, process development, or production include Microinnova, Lionix, MicroChemical Systems, and Syrris. Two others, Mikroglas Chemtech and Cellular Process Chemistry (CPC), are spin-offs of IMM, as is Ehrfeld Mikrotechnik BTS, now owned by Bayer Technology Services. Many firms are based in Europe, but Velocys, a spin-off of Battelle and Pacific Northwest National Laboratory, is in the U.S.
Many fine chemicals producers, like Bayer, have set up internal operations. Clariant created its Competence Center in MicroReaction Technology in early 2004 to offer the technology in its custom synthesis business. Sigma-Aldrich installed a CPC lab system last year to extend the chemistries it can perform. In March, Degussa started a new Project House on Process Intensification that embodies its work on microreactor technology and other tools that boost production efficiency.
Lonza, meanwhile, has been active in the field for about three years, concentrating on reactions where high selectivity is difficult. The firm hopes that its work will interest pharmaceutical customers. "One of the main drivers is getting better selectivity because we must really demonstrate an added value," says Dominique M. Roberge, project leader for microreactor technology at Lonza. He advocates a modular, multipurpose approach and stepwise implementation for greater chances of success.
Specific microreactors for specific reactions aren't practical, Roberge believes. "In pharmaceutical and fine chemicals, you really need a modular approach," he explains, where a few basic reactors are developed for the various physicochemical characteristics of different reactions. This would provide the flexibility and versatility needed to handle the large number of reactions employed by industry but with more limited resources.

THE IDEA IS to fit the production units to the chemical process and not the process to the units. At present, Roberge envisions a microreactor possibly replacing a batch reactor but not an entire production process. One consideration is the existing investment in infrastructure that companies have for running and working up reactions. Another is because "we don't yet have all the modules to deal with different phases," he says. "I think we are still a few years away from that."
Roberge and coworkers recently published a comparative economic analysis of micro- versus batch reactors (Chem. Eng. Technol. 2005, 28, 318). In their analysis, they also looked at the applicability of continuous and microprocesses to a collection of reactions used for fine and pharmaceutical chemicals. Of 86 reactions with well-characterized kinetics, Roberge says, "about half would benefit from a continuous process," and most of those from microreactor use.
But 63% of those reactions are not workable in a multipurpose microreactor, he explains, because of the presence of a solid, as might be found when a product or by-product precipitates out. Researchers are advancing the technology to deal with solids, but Roberge believes that such modules are not yet ready for routine, multipurpose work. Although the analysis indicates that only about 16 of the 86 reactions would work in microreactors, many more reactions that were avoided in the past might now be considered in process research.
Principal scientist Xini Zhang and coworkers at Johnson & Johnson Pharmaceutical R&D decided to look specifically at several reactions that are difficult to scale up in conventional reactors because of safety or other concerns but where the mass and heat-handling capabilities of microreactors make the reactions doable (Org. Process Res. Dev. 2004, 8, 455). These include highly exothermic reactions, reactions at elevated temperatures, ones with unstable intermediates, and those involving hazardous reagents.
"Microreactor technology is still very new," Zhang says, "but we see great potential because it works so well for these classes of reactions." Whereas the initial work at J&J focused on evaluating the technology, now, she adds, "we actually use the system to support ongoing projects."
Not only are previously taboo reactions accessible to chemists, but they can use them immediately to produce quantities of material. Process chemistry typically involves scaling up reactions from lab- to plant-sized reactors. "We'd usually try to get away from these types of reactions or spend a lot of time fine-tuning conditions to the stage where we can run safely," Zhang says. By simply running the microreactor longer, she explains, "you can go from milligram scale to kilogram scale, and there's often nothing in between that you have to do."
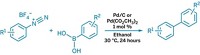
One test case from the J&J group is the ring-expansion of N-tert-butoxycarbonyl-4-piperidone with ethyl diazoacetate using boron trifluoride etherate. Applying conditions established for a 70-mg-scale batch reaction in a CPC Cytos microreactor, the researchers found that the reaction ran smoothly and safely under precise control to form the desired product in 89% yield, comparable with that in batch mode. The reaction requires only 1.8 minutes of residence time in the 35-mL microreactor at 10 ºC and has a throughput of 91 g of product per hour.
In addition to the inherent safety of small reaction volumes, microreactors also are opening up possibilities for chemistry with unstable intermediates. Zhang and her coworkers have studied the metal-halogen exchange step in the reaction of 3-bromoanisole with n-butyllithium to obtain 3-methoxyphenyllithium. The aryllithium compound is stable only at cryogenic temperatures and can be reacted in batch mode with (±)-2-[(dimethylamino)methyl]cyclohexanone to give, with high stereoselectivity, an alternative synthesis of the analgesic tramadol.
Because they have just one CPC microreactor system, J&J scientists made the aryllithium intermediate at -14 ºC using a 17-second reactor residence time and then reacted it in batch mode with cyclohexanone (as a model compound) to achieve an 87% yield and 54-g-per-hour throughput. With two successive microreactors, Zhang says, they could have fed the unstable intermediate directly into the second reactor to complete the synthesis sequence in a favorable continuous mode.

MEANWHILE, CPC has reported on similar lithiation and Grignard reactions with the added benefit of having multiple reactors available (Chem. Eng. Technol. 2005, 28, 408). Using a two-stage system, the optimized conversion of 3-bromoanisole to 3-methoxybenzaldehyde--by reacting the starting material first with n-butyllithium and the product of that reaction with dimethylformamide--took place at 0 ºC with a throughput of 59 g per hour and a yield of 88%. After 24 hours, they had made 1.4 kg of the final aldehyde safely and reproducibly.
Ube Industries and collaborators recently published work on Swern oxidations, reactions used extensively in making pharmaceutical intermediates (Angew. Chem. Int. Ed. 2005, 44, 2413). The oxidation using dimethyl sulfoxide (DMSO) is a versatile and reliable method for oxidizing alcohols to carbonyl compounds. Until now, however, the reaction using DMSO activated by trifluoroacetic anhydride (TFAA) has seen limited industrial use since it has to be conducted at or below -50 ºC to avoid the decomposition of an unstable cationic intermediate.
The partners--Ube, Japan's MCPT, and Kyoto University--developed a microscale tube reactor system for conducting Swern oxidations between -20 and 20 ºC. They used an IMM micromixer to combine the DMSO and TFAA; the solution was then passed through a stainless steel tube reactor and mixed with a solution of the alcohol in a second micromixer. This solution passed into a second reactor, was mixed with a triethylamine solution, and flowed through two final reactors before the product was collected.
The combination of precise temperature control, extremely fast and efficient mixing, and short residence times--a combined eight to 11 seconds for all four reactors--allows for the reactive intermediate to move to the next stage and react with the alcohol substrate before decomposing in 0.01 second. The system, the partners say, has allowed them to achieve equivalent or often significantly better conversions and yield for primary, secondary, cyclic, and benzylic alcohols at -20, 0, and 20 ºC, compared with lower temperature conditions.
To test for durability, the partners ran a cyclohexanol oxidation reaction for three hours at 20 ºC and saw consistent high conversion and product selectivity. "Our goal is to apply this technology to the process on a commercial scale," says Yoshinori Kawai, Ube's director for fine chemicals and active pharmaceutical ingredients (APIs). But first, "we will work on the pilot scale in the near future." The challenge, he adds, will be to improve microreactor performance and durability.
Research also is under way on other reactions involving unstable intermediates. Kyoto University scientists Kazuhiro Mae and Kunio Yube recently published work on the oxidation of aromatics with peroxides under severe conditions (Chem. Eng. Technol. 2005, 28, 331).
Similarly, the industrial production of phenylboronic acid is plagued by unwanted competitive reactions. Aryl and alkyl boron compounds are versatile building blocks used in Suzuki couplings to produce many valuable fine chemicals. Hessel, IMM coworkers, and collaborators at Clariant have used microreactors to produce the boronic acid with both selectivity gains and energy savings (Org. Process Res. Dev. 2004, 8, 511).
They investigated the formation of phenylboronic acid from addition of phenylmagnesium bromide to trimethylboronic acid ester in different microreactor systems. Although the industrial batch process is carried out at -40 to -50 °C, it is not very selective (65% yield of the desired product). Increasing the temperature only decreases the yield. With micromixers and tubular reactors, however, a maximum yield of 89% at 20 ºC is achieved because of better mixing efficiency and, presumably, isothermal processing.
Advertisement
The amounts of undesired products are reduced significantly, and a higher purity (99%) product is obtained compared with the conventional process (82%), the collaborators report. As a result, a costly distillation step could be eliminated and replaced with simple crystallization to achieve the desired product quality. In addition to these savings, energy costs are lower because the reaction no longer requires cooling.
Once a process is optimized--which needs to be done only once--moving it into production simply involves "scaling out" or "numbering up," which means running multiple microreactors in parallel to make the desired amount of material. Examples of pilot- and production-scale operations are being developed for industrial chemicals at Degussa, Velocys, FMC, and Dow Chemical (C&EN, Oct. 11, 2004, page 39). Detailed examples are less readily available in fine chemicals, say people knowledgeable in the field; Clariant's production of diazo pigments in a plant built by CPC is the most often cited.
Synthacon, a joint venture of CPC and ProBioGen, offers microreactor capacity on a contract basis. This venture has a CPC Cytos Pilot system, which uses 10 two-stage microreactor systems in parallel. Such systems cost about $1.5 million per reaction stage, compared with lab-scale systems that can range between $20,000 and $200,000. Synthacon also is building a commercial, large-scale, multiproduct plant on the Leuna industrial site near Leipzig, Germany. It is expected to start up by mid-2006.
Meanwhile, CPC has worked with GlaxoSmithKline to explore making existing products in pilot-scale continuous processes, says Thomas Schwalbe, a founder of CPC who has served as its chief executive officer. He heads a new operation called Micro-Reactor Systems Provider that initially will distribute CPC products. The operation also will look to supply other continuous-chemistry products and consult on batch-to-continuous conversions for fine chemicals and pharmaceutical intermediates. CPC has just introduced its Cytos-M system, a scaled-down version of the Cytos Lab system.
Schwalbe has also launched Acclavis, which will use microreactor technology for the combinatorial investigation of reaction pathways. It will then convert the information into process requirements and define the most cost-effective continuous- reaction equipment. Initial fields of interest are nanoparticle formation and specialty polymer processes, he says.
Although microreactors avoid many problems found in scaling up batch reactions, they still have some limitations. "There is no way of talking around the constraint of clogging that can occur," Schwalbe acknowledges. On the plus side, he adds, the chemistry can sometimes be adjusted to prevent it. For example, in lithiation reactions run at higher temperatures, he explains, "precipitation is a lesser issue because the reactions are very fast and many compounds stay in solution."
"You can aim to optimize a reaction such that the reagents are in the microreactor for only as long as it takes to do the conversion," he continues. And, he adds, you can adjust the flow rate so that compounds don't linger needlessly inside the microstructures. The hope is that this not only avoids precipitates from impurities, by-products, or the product itself but also gives the best selectivity.
In fast azo coupling reactions, for example, specially designed micromixers can ensure faster, complete mixing of reactants at the appropriate concentrations and flow rates. IMM and collaborators at Trustchem in Shanghai have recently used this method in the synthesis of an azo pigment (Org. Process Res. Dev. 2005, 9, 188). They also found that the microreactor process led to both smaller particles and a narrower size distribution in the final product and improved pigment properties.
FOULING and clogging are probably the most frequently raised concerns regarding whether current microreactors are stable and robust enough for consistent, continuous operation. In addition, experience in running them for long periods is limited, ICT's Löbbecke admits. One solution, common in continuous processing, he suggests, is to periodically introduce an automatic purging step to keep the systems clean and flowing.
Another major issue is corrosive media and chemical compatibility with associated equipment, such as pumps, and with microreactor materials. Microreactors are made from a variety of materials including stainless steel, Hastelloy, glass, silicon, polymers, and ceramics (C&EN, June 16, 2003, page 36). "There's a lot of data concerning corrosion resistance, but it's all for macroscale reactors and is in terms of millimeters per year," Löbbecke explains. "That's not a help--if you lose a millimeter in a microreactor, you'll actually lose the entire reactor."
Robustness is an issue that Lonza wants to address with a continuous small-scale plant (CSSP) capable of rapidly producing kilogram quantities for preclinical and Phase I studies. "We plan that it will work under current Good Manufacturing Process conditions," Roberge says. Now under construction, Lonza's CSSP will use one microreactor to effectively replace a 160-L batch vessel.
Because some APIs and drugs are already produced via continuous processes, microreactor setups are not expected to have major regulatory consequences. What is more uncertain is how regulators will view products produced in parallel. The Food & Drug Administration, for example, does not allow batches to be mixed, drug researchers tell C&EN, and so it's unclear whether parallel production will be viewed as one batch through multiple channels or several batches being mixed.
Microreactor R&D has focused largely on reactions involving gas and liquid phases and, to a lesser extent, solid phases. As is the case for many of the industrial chemical projects, Degussa and its partners have been working on gas-phase heterogeneous catalyzed reactions using very large reactors having microstructures (Chem. Eng. Technol. 2005, 28, 459). "The processing of solids in microstructured devices is still an unresolved issue," says Henrik Hahn, director of Degussa's Process Intensification Project House.
Homogeneous and heterogeneous catalysis has become a desirable route for the production of fine chemicals and pharmaceuticals. Researchers at the University of Hull, in England, for example, have studied both the Kumada-Corriu and Suzuki reactions in microreactors. In the latter case, the process was modified to immobilize the palladium catalyst between microporous silica frits. This resulted in yields comparable with those obtained under homogeneous batch conditions, with the added benefit of negligible levels of catalyst in the product (Sens. Actuators, B 2000, 63, 153).
Haswell and Watts, along with Esteban Pombo-Villar of Novartis, have explored a number of pharmaceutically relevant reactions in microreactors, including Michael additions, aldol condensations, heterocyclic reactions, and multistep peptide synthesis. Recently, Watts and other coworkers have expanded work with glass microreactors and electroosmotic flow (EOF) to solid-supported, continuous-flow synthesis (Org. Process Res. Dev. 2004, 8, 942).
There have been very few reports of solution-phase organic synthesis using solid-supported reagents or catalysts in microreactors, the researchers say. One challenge is incorporating the solid material into the microreactor and not destroying it while doing so. Approaches include coating reactor walls, trapping catalyst particles in or tethering them to microstructures, and packing microchannels or capillaries. Once successful, however, these approaches can offer many potential benefits in continuous-flow systems.
"We find that reactions are quicker in a microfluidic system because you are physically pushing the molecules through a packed bed such that the surface-to-volume ratio is high and there really is contact with the catalytic surface," Watts explains. The catalyst must be tightly packed to avoid flow around, rather than through, the material. Using EOF is advantageous, he adds, as it is independent of particle size and offers more reproducible low flow rates than do pressure-driven systems.
BENCHMARKING
Examples Abound For Reactions That Have Worked In Microreactors
Acrylate polymerization
Addition of organometallics to carbonyl compounds
Aldol/carbanion chemistry
Amides from amines and acid chlorides
Brominations of toluene, 3-nitro-toluene, and thiophene
Chlorination of acetic acid
Cumene hydroperoxide rearrangement
Diazomethane conversion
Diazotization and diazo coupling
Diels-Alder reaction
Dehydrations
Electrochemical synthesis
Enamine synthesis
Enzymatic reactions
Epoxidation reactions
Esterification of pyrenyl-alkyl acids
Fluorination of aromatics and aliphatics
Grignard chemistry
Hantzsch reaction
Heck reactions
Hydrogen/oxygen reduction to hydrogen peroxide
Hydrogenations, including aromatic nitro compounds
Hydrolysis of benzal chlorides to aldehydes
Knoevenagel reactions
Kolbe-Schmitt synthesis
Kumada-Corriu reaction
Methylation of aromatics
Michael addition
Newman Kwart rearrangement
Nitration of aromatics and aliphatics
Oxidations of aromatics, ethanol, ethylene oxide, maleic anhydride
Peptide synthesis
Photochemical synthesis
Sonogashira coupling
Suzuki coupling
Wittig olefinations
IN CONTRAST to a batch reactor, the catalyst is always in excess over the reactants, Watts says, and he believes this should contribute to higher turnover. "And you don't have to remove the catalyst from the reaction mixture because it is immobilized," he adds. "All you have to do is remove the solvent, and you have a pure product."
The Hull group has performed Knoevenagel reactions to test this. Using piperazine immobilized on benzyl chloride-functionalized silica packed in a capillary, they reacted ethyl cyanoacetate and 4-bromobenzaldehyde in acetonitrile to produce ethyl 3-(4-bromophenyl)-2-cyanoacrylate. They found that, in contrast to pressure-driven setups, the system generated reproducible high conversions with different aldehyde derivatives.
Advertisement
Hydrogenations are another class of reactions being studied in microreactors. Usually highly exothermic, hydrogenations account for 10-20% of all reactions in the pharmaceutical industry, points out Ronald S. Besser, Stevens Institute chemical engineering professor and NJCMCS codirector. NJCMCS and industrial partners Bristol-Myers Squibb and Lucent Technologies, under a $2.4 million Department of Energy grant, have been working since late 2003 on developing microreactors for catalytic hydrogenation reactions.
They have successfully tested a microfabricated silicon reactor, just a few square centimeters in size, on a model reaction--the hydrogenation of o-nitroanisole to o-anisidine--and will soon move to proprietary molecules, Besser says. The microreactor handles both the gas and liquid phases, while immobilizing Pd/C catalyst particles in thousands of small microstructured traps.
The design goals include adequate catalyst loading compared with what one might get with wall coatings and better material transport and lower pressure drops than might occur in packed beds. But another major goal is to create a system that will let researchers readily screen available commercial catalyst powders while preserving the advantages of the microscale reaction environment, Besser adds.
In addition to the hydrogenation project, Bristol-Myers Squibb has filed for patents on a method for making glycosides using a noncryogenic process. It includes lithiating an aromatic reactant having a leaving group in a microreactor to form a lithiated anion species. This anion species is then coupled with a carbonyl-substituted reactant to form a glycoside. The lithiating and coupling steps are rapid and highly exothermic. The approach, according to the patent claims, offers reduced by-products, improved yields, and decreased costs of manufacture.
Glycoside formation using microreactors has also been the focus of a team of researchers at Massachusetts Institute of Technology and the Swiss Federal Institute of Technology (ETH), Zurich (C&EN, Feb. 7, page 11). In this instance, they used an MIT-developed microreactor system to quickly optimize yield and selectivity in a variety of glycosylation reactions using very little starting material.
By screening reaction variables such as composition, flow rates, and temperature, the MIT-ETH team also gained information on reaction paths and kinetics. In past work, they have explored a variety of complex and difficult reactions and have also shown that their device is amenable to integrated reaction monitoring using mass spectrometry, gas chromatography, infrared spectroscopy, and other common types of spectroscopy.
Other researchers and microreactor developers are combining microreactor and other technologies, such as high-throughput experimentation (see page 54) for process optimization and in situ analytics (see page 63) to monitor reaction conditions and product quality. For example, Mikroglas and Syrris both sell systems with on-line analyzers, while Lionix offers various in situ reaction sensors. Ehrfeld and FAMOS systems both have optical interfaces or flow-through modules for in-line analytics.
Syrris offers a system called AFRICA (Automated Flow Reaction Incubation & Control Apparatus) that it created with partner GlaxoSmithKline. With it, researchers can run a sequence of microreactions under multiple conditions for process optimization. Material can be diverted to an associated high-performance liquid chromatography system for immediate analysis. In late 2004, Syrris began a collaboration on multistep synthesis with University of Cambridge chemistry professor Steven V. Ley, whose group has studied homogeneous reactions with immobilized reagents under flow conditions.
Jennifer L. Rutherford, a scientist in chemical R&D at Pfizer, has been using a customized CPC Sequos system that combines a flow microreactor with automated multiple reagent feeds and a UV detector. The combination, she says, offers the potential for high-throughput screening of microreactions by changing both physical and chemical parameters. CPC's standard Sequos (for sequential organic synthesis) system targets combinatorial applications, Schwalbe notes.
In addition to the modular FAMOS system for laboratory and R&D purposes, Fraunhofer ICT has developed an automated microreaction system, called AuMµRes, with Siemens and Merck KGaA. Descriptions of both systems can be found in a recent article (Chem. Eng. Technol. 2005, 28, 484). "AuMµRes was originally designed for screening parameters in very fast, strongly exothermic, and highly corrosive nitration reactions," Fraunhofer ICT's Löbbecke says.
The AuMµRes system combines microreactors with microstructured flow-through sensors and analytical interfaces for monitoring reactants and process conditions. "You can change and screen parameters very systematically and gather information in a very short time to optimize a reaction and see what are the main drivers to improve selectivity, increase yield, or suppress side reactions," Löbbecke explains. The system is also capable of "small-scale production in the kilogram-per-day range," he adds.
The information gained from working with microreactors might prove useful in scaling out or even scaling up a process. "In some cases, you are interested in gathering information about your chemical reaction and then using the benefits that come from miniaturization," Löbbecke says. But data about reaction mechanisms and kinetics can also be used to redesign a macroscale process, whether it is continuous or batch, he adds.
Clearly, under the right conditions and for the right reaction, microreactors can offer better selectivity, improved yields, increased process control, greater safety, faster scale-up, flexible production, and the opportunity to tap into previously avoided or novel chemistries, experts say. These achievements all meet the needs of the fine and pharmaceutical chemicals industry and are strong incentives for adopting the technology.
Challenges remain, however. Process chemists need to become more familiar with the technology before they implement it further. Operating and capital costs must be balanced against existing investments and the potential benefits of the new technology. Microreactors work best with relatively fast reactions and, today, with those requiring low throughput for making high-value materials.
Even the strongest proponents of microreactor technology advise prudence. In one of their review papers, the Hull and IMM researchers state: "Since the learning curve is large, the market is conservative, and the tool is not the solution to anything, it becomes increasingly apparent that 'micro' should be only placed where it is needed and not where it may be technically feasible."





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter