Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Complexity of Tamiflu Manufacturing May Hamper on Demand Production
by Amanda Yarnell
August 29, 2005
| A version of this story appeared in
Volume 83, Issue 35
Business
PROCESS CHEMISTRY
From the sourcing of the raw material to the production of capsules, it takes a full 12 months to make Tamiflu, according to Martin Karpf of Swiss drug giant F. Hoffmann-La Roches synthetic and process research group. Manufacture of the oral antiviral, thought to be the most promising weapon against avian influenza currently available, involves time-consuming routes to the starting material and steps that require potentially hazardous azide chemistry.
The 10-step commercial route uses the natural product (–)-shikimic acid as a starting material. This precursor is converted into a diethyl ketal intermediate, which is reductively opened to give a 1,2-epoxide. This epoxide is then converted into Tamiflu via a five-step reaction sequence involving three potentially toxic and explosive azide intermediates. This optimized route gives Tamiflu in 35% yield.
Initially, research quantities of (–)-shikimic acid cost more than $50 per gram, Karpf notes. This precursor simply was not available on the world market in large amounts, he says.
Needing to obtain a cheaper and more reliable supply of the starting material, Roche turned to Michigan State University chemist John W. Frost, whose lab had developed a strain of Eschericia coli that overproduces (–)-shikimic acid when fed glucose. Within a year and a half, Roche had figured out how to grow these bacteria on a commercial scale and determined how to extract and purify the (–)-shikimic acid.
An efficient route to extraction of (–)-shikimic acid from Chinese star anise also has become available, Karpf notes. Roche now relies on both fermentation and extraction to obtain ton quantities of (–)-shikimic acid, he tells C&EN. But with either method, isolation and purification of (–)-shikimic acid remains time-consuming.
The dependence on azide chemistry to convert the epoxide intermediate into a 1,2-diamine also poses a bottleneck to Tamiflu production. Safety and economic reasons have forced Roche to rely on a contract firm that specializes in azide chemistry to perform this sequence, Karpf says.
Roche has explored ways to speed up production (Chimia 2004, 58, 621). It has developed an azide-free allylamine route from the epoxide to Tamiflu. It has also crafted routes that dont rely on (–)-shikimic acid: a Diels-Alder-based one that uses furan and ethyl acrylate as starting materials and another that relies on catalytic hydrogenation of an isophthalic acid derivative followed by enzymatic desymmetrization. And Frost has begun to develop a microbial synthesis of aminoshikimic acid, which could reduce the need for azide chemistry if used as a starting material (Org. Lett. 2004, 6, 1585). So far, however, none of these alternatives has measured up to the commercial route in terms of cost and efficiency.



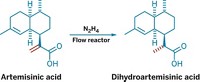
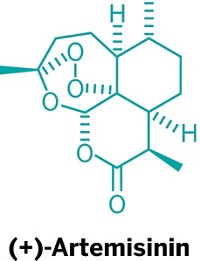

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter