Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Inorganic Chemistry: the Next Generation
Symposium honors outstanding young inorganic chemists as they get set to launch their careers
by Stephen K. Ritter
October 3, 2005
| A version of this story appeared in
Volume 83, Issue 40
ACS MEETING NEWS
Mature fields of science all have their share of worriers who fret about the future of their discipline. Chemistry is right at the top of the list. Concerns have been widely expressed about the quality of new chemistry graduates and if enough new chemists are in the pipeline to keep the U.S. at the forefront of science and technology.
But as activities at the recent American Chemical Society national meeting in Washington, D.C., showed, inorganic chemists can take some comfort in knowing that their next generation of practitioners is already making significant contributions to the field. One event, sponsored by the Division of Inorganic Chemistry, was a first-time symposium featuring the research of eight select graduate students and postdoctoral researchers who were named Inorganic Young Investigators.
The Inorganic Division's leadership believed that "showcasing the science of some of the most promising young investigators in a major symposium would serve several useful purposes," noted chemistry professor Clifford P. Kubiak of the University of California, San Diego, who is the current division chair. "It's a goal worth attaining for our students and postdocs. It features really current science as part of our programming at ACS meetings. And it's a way to check out rising stars in inorganic chemistry."
Chemistry professor T. Don Tilley of the University of California, Berkeley, first suggested the idea for the symposium when he was division chair in 2003. "We wanted to recognize the contributions and dedication of graduate students and postdocs who come to ACS meetings to present their work," Tilley commented. "One reason the Inorganic Division is so successful and has so many papers presented at the national meetings is because of these students."
The symposium was advertised to members of the Inorganic Division and open to students and postdocs who had not yet accepted their first independent position. More than 50 nominations were collected by a committee made up of the chairs-elect of the Inorganic subdivisions-Bioinorganic, Nanoscience, Organometallic, and Solid-State & Materials. Two winners were selected from each subdivision, and each award recipient received $500, a plaque, and the opportunity to give a 20-minute presentation on his or her research.
"We were very pleased with the symposium, and we plan to do it again next year, and every year thereafter," Kubiak said.
The following are vignettes about the eight Inorganic Young Investigators and their research:
Robin T. Macaluso, a postdoc at Argonne National Laboratory, gave a lecture on how crystal structure can affect local and itinerant electronic behavior in intermetallic magnetic and superconducting materials. She carried out the work while a graduate student in Julia Y. Chan's lab at Louisiana State University.
Macaluso used X-ray diffraction along with magnetic and electronic property measurements to study Ce2PdGa12, Ce2MIn3n+2 (M = Co, Rh, or Ir; n = 1 or 2), and other intermetallic systems made by heating stoichiometric amounts of the pure elements at high temperatures (Chem. Mater. 2003, 15, 1394). In particular, she has investigated structural trends that result from the presence of f electrons in the electronic structure of these materials, and how certain structure types favor “heavy-fermion” superconductivity. Heavy-fermion materials are those in which conduction electrons have a greater effective mass because they strongly interact with the localized magnetic moments of f electrons in the crystal lattices.
Macaluso was one of several award winners who commented on the importance of education and outreach during their presentations. "As a former high school chemistry and physics teacher, I realize that science is a difficult subject to relate to students," Macaluso said after the meeting. She believes it's important to develop exciting demonstrations and lessons to teach students the importance of chemistry, and for everyone to become involved with science education.
"But it is a unique experience for young scientists to do so,"she added."K through 12 students relate to young investigators. They see that becoming a scientist is an attainable goal, and they are less intimidated to inquire about science or the science career path."
Timothy A. Jackson described his doctoral research in which he combined spectroscopy and computational chemistry to study the reactivity of iron and manganese superoxide dismutases. Jackson was a graduate student in Thomas C. Brunold's group at the University of Wisconsin, Madison, and he is currently a postdoc working with Lawrence Que Jr. at the University of Minnesota, Twin Cities.
Superoxide dismutases are metalloenzymes that protect plants and animals from oxidative tissue damage caused by superoxide radical anions (O2⋅-), Jackson explained. The radicals are produced from oxygen as a by-product of photosynthesis or respiration, and the metal-specific enzymes in turn catalyze disproportionation of the radicals to O2 and H2O2.
Jackson and his colleagues focused on understanding "second-sphere effects" in the enzyme's active site. They found that a hydrogen-bonding network formed by amino acid residues and a coordinated water solvent molecule surrounding the active site can play a critical role in modulating properties that control enzyme activity (Acc. Chem. Res. 2004, 37, 461).
"As a graduate student, I think it's sometimes a little tricky to gauge how well you're doing in the larger scheme of things-that is, how does your graduate education compare with those at other universities?" Jackson told C&EN. "Receiving this award has shown me that my education has been pretty good and has in turn reassured me that I'm on the right track in terms of my academic aspirations."
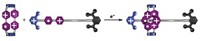
Dorothea Fiedler recently graduated from UC Berkeley and is planning to pursue an academic career. Her research was part of a joint project with chemistry professors Kenneth N. Raymond and Robert G. Bergman that focused on using self-assembled supramolecular cages as catalytic nanoscale reaction vessels. Much like the active site of an enzyme, the structure of the cage assembly provides an environment for controlled reactivity and selectivity.
Fiedler and her colleagues focused on a gallium cluster, [Ga4L6]12-, where the gallium atoms occupy the vertices of a tetrahedron and are linked along the six geometric edges of the tetrahedron by benzamidonaphthalene ligands. They took two different approaches with the assemblies: utilizing the space-restrictive cavity as a catalyst itself, or encapsulating catalytically active transition-metal complexes as guest molecules, specifically ruthenium cyclopentadienyl half-sandwich complexes (J. Am. Chem. Soc. 2004, 126, 3674).
One example she described was the use of the cavity as a catalyst to effect the aza-Cope rearrangement on various enammonium cations. The cations are bound in the gallium cavity in a reactive conformation that accelerates the rate of rearrangement to iminium cations. The rearranged cations are released from the cavity and hydrolyzed to unsaturated aldehydes (Angew. Chem. Int. Ed. 2004, 43, 6748).

Walter F. Paxton, a graduate student in Ayusman Sen's group at Pennsylvania State University, University Park, has designed nanorods consisting of platinum and gold segments that spontaneously move in hydrogen peroxide solution (C&EN, Feb. 21, page 33). Several groups of scientists are designing this type of chemical system that can mimic the locomotion of objects found in nature, Paxton noted. "The idea is that a small particle with a catalyst on it could harvest chemical energy from its environment and convert that into a useful mechanical force," he said.
The 370-nm-diameter rods, consisting of 1-µm-long gold and platinum segments, are formed by electrodeposition on alumina substrates. Catalytic decomposition of H2O2 and formation of O2 on the surface of the rods set them in motion, Paxton explained. The rods move at speeds up to about 10 µm per second, a rate similar to that of flagellar bacteria. Paxton, Sen, and their collaborators, including Thomas E. Mallouk at Penn State, developed a mathematical model to investigate the mechanism of the motility, which they ascribe to an "interfacial tension gradient" between the rods and the solution that is continuously reestablished as the rods move.
Other work, with fellow graduate student Timothy R. Kline, includes nanorods with added nickel segments that can be controlled by a magnetic field. Real-world applications are still a long way off, Paxton said, but molecules or particles potentially could be attached to the rods to create tiny machines that might someday be used as drug delivery vehicles or as nanobots to keep arteries unclogged. He is planning for a career in industrial R&D.

Theodore A. Betley presented his Ph.D research on the redox chemistry of iron that was carried out at California Institute of Technology in the lab of Jonas C. Peters. "Iron is an interesting metal because it plays a central role in many biological and industrial processes," Betley said. "With the right ligand platform, we can access five different oxidation states with iron through substitution of a single ligand. This redox flexibility becomes essential when describing iron's potential role in certain chemical transformations, such as dinitrogen activation."
With tris(phosphino)borate ligands, which coordinate to iron through the three phosphorus atoms, Betley was able to show that tetrahedral iron can coordinate dinitrogen, diazenido, and nitride ligands at the fourth coordination site. Iron in these complexes assumes different oxidation states, from Fe(0) to Fe(IV), to accommodate reduced or functionalized nitrogen species that could be part of the enzymatic nitrogen-fixing pathway that plants use to form ammonia (J. Am. Chem. Soc. 2004, 126, 6252). Betley currently is a postdoc in Daniel G. Nocera's group at Massachusetts Institute of Technology.
Elizabeth M. Nolan of MIT gave a talk on the development of fluorescent zinc sensor molecules that could be used for in vivo optical imaging. The work is part of her ongoing graduate research in Stephen J. Lippard's group. The water-soluble fluorescein-based chemosensors selectively bind free zinc(II) ions in biological samples, resulting in a significant increase in the molecules' fluorescence, she explained.
Zinc ions exist in high concentrations in brain tissues and are thought to be important for controlling neurotransmission, Nolan said. Uncontrolled zinc ion concentrations may play a role in the development of Alzheimer's disease or in neuron damage following a brain injury or stroke. Developing zinc imaging agents with high sensitivity and selectivity are thus needed to move forward with research, she noted.
One family of molecules developed in Lippard's lab, called "Zinpyr" sensors, has a fluorescein core with aminopyridine substituents (Chem. Biol. 2004, 11, 203). Nolan has worked on these compounds and has developed the related family of "Zinspy" sensors that contain sulfur-bearing groups, such as thioethers (Inorg. Chem. 2004, 43, 8310). She also has synthesized "QZ" systems that contain aminoquinoline substituents.
Most of these compounds provide up to about a 10-fold enhancement in fluorescence upon zinc binding, but one of the QZ compounds has a 150-fold fluorescence enhancement, she said. The researchers are in the process of testing the sensors to track zinc ions in cultured tissues.

Tamara D. Hamilton's graduate research in Leonard R. MacGillivray's group at the University of Iowa involved synthesis of tetrapyridylcyclobutane ligands that self-assemble into molecular frameworks when treated with transition-metal ions. These assemblies can take on various structures, including 2-D polygons and 3-D polyhedrons, that could serve as hosts to stabilize chemical intermediates, mediate chemical reactions, or be useful building blocks for larger molecular-scale devices (Cryst. Growth Des. 2004, 4, 419).
Preparation of the metal-organic assemblies was designed to mimic template-directed processes in nature, such as DNA-directed synthesis of proteins, Hamilton noted. The ligands are synthesized from resorcinol and dipyridylethylene by a solid-state [2 + 2] photodimerization reaction (J. Am. Chem. Soc. 2002, 124, 11606). Treating the ligand with Cu(II) ions, for example, forms a polyhedron structure made up of six copper ions and six ligand molecules.
The ligand is unsymmetrical because it contains both 2-pyridyl and 4-pyridyl groups, she added. But this quirk provides the advantage that one set of pyridines can be “turned off” during the self-assembly process, allowing access to a wider variety of assembly geometries. Hamilton currently is a postdoc in James D. Wuest's group at the University of Montreal. She plans an academic career.
Matt Law, a graduate student in Peidong Yang's group at UC Berkeley, has focused on the chemistry and physics of inorganic nanowires. "I've tried to show the many different ways that SnO2 and ZnO nanowires can be useful, including as chemical sensors, nanomechanical elements, waveguides and photonic devices, lasers, and solar cells," he said.
Advertisement
Law described work to fabricate solar cells based on dense arrays of single-crystalline ZnO nanowires that are either coated with a monolayer of dye molecules or filled by a semiconducting polymer. The oriented nanowires provide a more direct pathway for charge transport in the devices compared with nanocrystalline films or polymer blends with disordered 3-D topologies that typically are used in nanostructured solar cells, he said.
In one example, Law and his colleagues showed that replacing the traditional TiO2 nanoparticle film in a dye-sensitized solar cell with a dye-coated ZnO nanowire array increases charge collection efficiency (Nat. Mater. 2005, 4, 455). In another example, Law and his coworkers describe a general method to make oriented ZnO arrays (Nano Lett. 2005, 5, 1231). Arrays of uncoated nanorods-shorter versions of the nanowires--made by this method can be filled with a semiconducting polymer, such as poly(3-hexylthiophene), to produce functioning devices.
"Being named a Young Investigator is certainly a great surprise and honor," Law told C&EN. "I hope this experience will help to propel me to a position where I can make important contributions to the rational design of nanostructured devices for energy conversion and storage."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter