Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Protein's Lipid Coat Revealed
High-resolution structure of membrane protein captures its lipid environment
by Amanda Yarnell
December 5, 2005
| A version of this story appeared in
Volume 83, Issue 49

STRUCTURAL BIOLOGY
Relatively little is known about how membrane proteins interact with the lipids that surround them because structural studies typically fail to capture these proteins' lipid environment. Now, a high-resolution structure of a membrane-embedded water channel protein has opened a window to how lipids pack around membrane proteins (Nature 2005, 438, 633).
The key to an effective membrane is to get the packing of the lipids and proteins right, explains Anthony G. Lee of the University of Southampton, England, in an accompanying Nature commentary. The new structural data clearly illustrate how this packing is achieved.
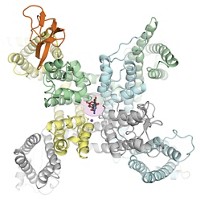
Thomas Walz of Harvard Medical School and coworkers captured the 1.9- structure of aquaporin-0 (AQP0) immersed in an artificial lipid bilayer. AQP0 is a water channel that is the most abundant membrane protein in the lens of the eye. They used electron crystallography, a well-established technique that employs the beam of an electron microscope to produce diffraction patterns from frozen two-dimensional crystalline arrays. It's particularly powerful for imaging membrane proteins, Walz says, because it allows them to be imaged in their native environment, the lipid bilayer.
Previously, crystal structures of a number of membrane proteins have revealed a small number of specifically bound lipids. But the AQP0 structure, which Walz solved with the help of Tamir Gonen, Yifan Cheng, and Stephen C. Harrison of Harvard and Yoshinori Fujiyoshi of Kyoto University in Japan, provides a very different picture.
In the structure, a thin shell of lipid bilayer surrounds the hydrophobic midsection of the protein. The lipid is dimyristoylphosphatidylcholine, a non-endogenous lipid with a zwitterionic head and two 14-carbon fatty acid tails. The lipid molecules stack tail-to-tail, with their fatty acyl chains packed tightly around the protein's bumpy midsection. The resulting shell provides a uniform surface against which the rest of the lipids in the membrane can pack. Their heads interact with charged side chains on the hydrophilic portions of the protein that would typically be at the membrane-water interface.
Walz suggests that the lipid-protein interactions observed in the structure may be representative of the nonspecific interactions that occur between any membrane protein and the natural lipids in cell membranes. The lipid used in this study, however, is not found in natural membranes, so it remains to be tested whether endogenous lipids make similar interactions with AQP0, he admits.
Eventually, structural biologists hope to determine at a molecular level the arrangement of proteins in cell membranes, notes James Allen of Arizona State University. He calls the new study an exciting advance toward that goal.
Mark S. P. Sansom of the University of Oxford adds that from a chemical perspective, this detailed picture of lipid-protein interactions should help us sort out the design rules of how to lock a macromolecule into a membrane.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter