Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Flip-flopped Structure
Finding suggests mechanism for how protein pumps drugs out of cells
by Celia Henry Arnaud
January 2, 2006
| A version of this story appeared in
Volume 84, Issue 1

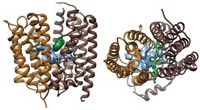
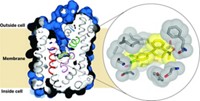
Proteins that pump drugs out of bacterial cells contribute to the problem of multidrug resistance in diseases such as tuberculosis. New research findings may help lead to a solution to this problem. Geoffrey Chang and coworkers at Scripps Research Institute report the X-ray crystal structure at 3.7-Å resolution of one of these proteins in action—the EmrE protein from Escherichia coli bound to the arsonium analog of tetraphenylphosphonium, one of the drugs EmrE transports (Science 2005, 310, 1950).
EmrE makes bacteria resistant to tetracycline, ethidium, and other cationic antibiotics. Developing inhibitors of such protein pumps could make old drugs effective again.
This membrane protein is a homodimer made of two chemically but not structurally identical polypeptides that align themselves in an inverted, antiparallel fashion. Although these subunits have the same amino acid sequence, they adopt different conformations, making the protein asymmetric.
Each subunit is composed of four helices. The arrangement of the first three helices is nearly identical in each subunit; the fourth helix, however, is packed differently. The difference between the four helices is "the structural basis for the asymmetry and also explains how the [drug] transporter could have a function that's unidirectional," Chang says.
Although this asymmetric arrangement was predicted by a cryo-electron-microscopic analysis of 2-D EmrE crystals, "this is the first convincing demonstration of the phenomenon," says Chris Tate, a structural biologist at the MRC Laboratory of Molecular Biology, Cambridge, England.
The earlier cryo-EM work suggested a similar but not identical structure for EmrE. "I think they're seeing the transporter in one conformation, and we're seeing it in another," Chang says. "That's great for biology because then you can actually form a biological story between the two." In the current paper, Chang and his coauthors write, "interconversion of the EmrE transporter between these two (and perhaps other) conformations may drive drug transport."
The X-ray structure reveals the location of two glutamates that have previously been shown through biochemical experiments to be essential for drug efflux. These glutamates have previously been proposed to contact the drug simultaneously, but Chang believes that the drug is handed off from one glutamate to the other. "It's going to take some time to figure this out from a biochemical aspect," he says. "The structural information is very compelling for this type of mechanism."
"From a biological perspective, the most remarkable contribution of this paper is that it unambiguously shows that functional antiparallel homodimers of membrane proteins are indeed possible," says Iban Ubarretxena-Belandia, a structural biologist at City University of New York. "Although such an arrangement might not be generalized in nature, it raises fundamental questions for membrane protein biosynthesis and structure."
RETRACTION
The paper described in this article has since been retracted (Science, DOI: 10.1126/science.314.5807.1875b).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter