Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Natural Product Synthesis On The Fly
Multistep continuous reaction shows the power of automated flow synthesis
by Stephen K. Ritter
March 6, 2006
| A version of this story appeared in
Volume 84, Issue 10

Combining the best techniques for automated chemistry with the green chemistry concept of consolidating or eliminating reaction steps, Steven V. Ley and coworkers at the University of Cambridge have accomplished the first multistep synthesis of a complex natural product in a continuous-flow reaction (Chem. Commun., published online Feb. 15, dx.doi.org/10.1039/b600382f). Their intensified process for synthesizing the widely studied alkaloid oxomaritidine may be a milestone for the future of pharmaceutical and fine chemicals production.
Ley's group is known for its polymer-supported reagents, catalysts, and cleanup scavengers that are used in the synthesis and purification of compounds to create combinatorial libraries and individual compounds in one-pot reactions. The work has evolved to include microfluidic glass reaction chips and small columns containing the immobilized reagents that can be used in series to carry out seamless multistep reactions.
In late 2004, Ley began a collaboration with Syrris, a U.K.-based microreactor company, to explore multistep syntheses using one of the company's systems. This union led to the successful procedure to make oxomaritidine.
The benefits of multistep continuous reactors employing immobilized reagents and microfluidic devices are considerable. The reactors allow better control over synthesis conditions, make more efficient use of starting materials, can readily recycle chemicals, and are able to circumvent or eliminate isolation and purification steps common in batch processing. Overall environmental and economic gains are possible by reducing solvent use, energy consumption, waste generation, labor costs, and time.
"This is really exciting research," comments John C. Warner, director of the Center for Green Chemistry at the University of Massachusetts, Lowell. "We have traditionally tried to compartmentalize various reaction stages. Ley's group has been able to escape these confines and design a process that elegantly strings the steps together. I see this work as a declaration that complex synthetic schemes in general can be redesigned for flow processing."
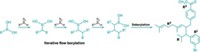
The Cambridge process, which involves seven synthetic steps, can generate gram quantities of oxomaritidine in 40% overall yield and 90% purity in only one day. Key steps include trapping previously prepared 4-(2-azidoethyl)phenol with a polymer-supported phosphine on a column and subsequently coupling it with 3,4-dimethoxybenzaldehyde, which is prepared in a separate channel.
The resulting imine intermediate is sequentially converted to an amide in steps that include changing solvents and a palladium-mediated hydrogenation. The hydrogenation takes place in a flow-through unit developed by Thales Nanotechnology, Budapest, Hungary. In the final stages, the third ring of oxomaritidine is formed and the amide is cleaved, promoting a bridging 1,4-addition to give the end product.
"We can envisage many exciting opportunities for machine production of chemical entities using these on-demand techniques," Ley says. "Processes can be scaled down or scaled up, creating possibilities for the preparation and online screening of compounds for biological or materials properties, or for their efficient production."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter