Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Enzyme plays dual role in Alzheimer's
March 27, 2006
| A version of this story appeared in
Volume 84, Issue 13
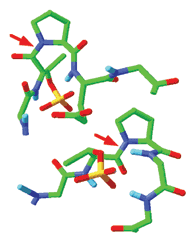
Tau proteins and amyloid-β peptides aggregate to form tangles and plaques in the brains of Alzheimer's disease patients. Back in 2003, Kun Ping Lu of Harvard Medical School showed that the enzyme Pin1 limits formation of tau tangles. Now, Lu and colleagues have found that Pin1 also curbs formation of amyloid plaques (Nature 2006, 440, 528). The group found that Pin1 binds to a phosphorylated threonine/proline segment of amyloid precursor protein in a way that favors its conversion from a cis (top) to a trans (bottom) conformation (red arrow indicates isomerized bond). The trans conformation of amyloid precursor protein is then clipped by other cellular enzymes into fragments useful for neuronal growth and survival. The cis conformation, on the other hand, is processed into fragments that include toxic amyloid-β peptides. The findings suggest that "lack of sufficient Pin1 enzyme may be a key culprit in the onset of Alzheimer's disease," Lu says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter