Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Aspirin transmogrification
April 3, 2006
| A version of this story appeared in
Volume 84, Issue 14
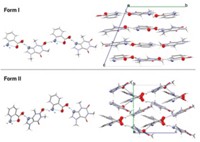
The Science & Technology concentrate about a newly discovered aspirin polymorph triggered a lot of nostalgia (C&EN, Nov. 21, 2005, page 50). I tried to explain in a paper the discrepancies associated with the previously published properties of the acetylsalicylamide isomers (Tetrahedron 1967, 23, 863). I determined that O-acetylsalicylamide rapidly and irreversibly rearranges under a variety of conditions to the N-acetyl isomer. Depending on how the N-acetyl isomer was prepared, during melting observed under a microscope along with differential thermal analysis, it was shown to exist in two different needle forms as well as rectangular plates over a fusion-solidification temperature range of 137-148 oC. Perhaps the salicylic acid system has a general proclivity to transmogrify.
Arnold J. Gordon
Greenwich, Conn.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter