Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
All That Glows
Bioluminescence provides practical applications while still remaining a mystery
by Rachel Sheremeta Pepling
April 3, 2006
| A version of this story appeared in
Volume 84, Issue 14




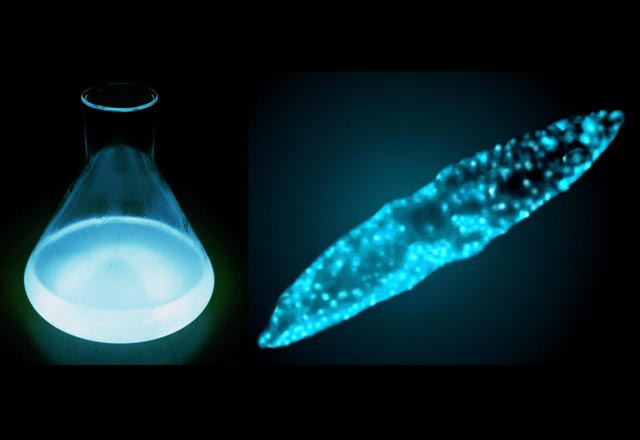
Three young boys run into the darkened exhibit space. They stop in their tracks in the middle of the hall and stare at the glowing displays and video screens of strange luminescent creatures looming above them. "This is so cool!"
Geared mainly toward kids, an exhibit currently at the Florida Museum of Natural History in Gainesville called "Glow: Living Lights" guides visitors through a brief overview of the fascinating world of bioluminescence. Glowing tanks of swirling dinoflagellates, monitors displaying rotating ribbon structures, and jars of preserved deepwater organisms provide a world of illumination for the intrigued boys.
But beyond the realm of museum exhibits, bioluminescence has captivated chemists and biologists alike since Johns Hopkins University became a hotbed for firefly bioluminescence research in the 1950s. At least for fireflies, the basic reaction is now well understood.
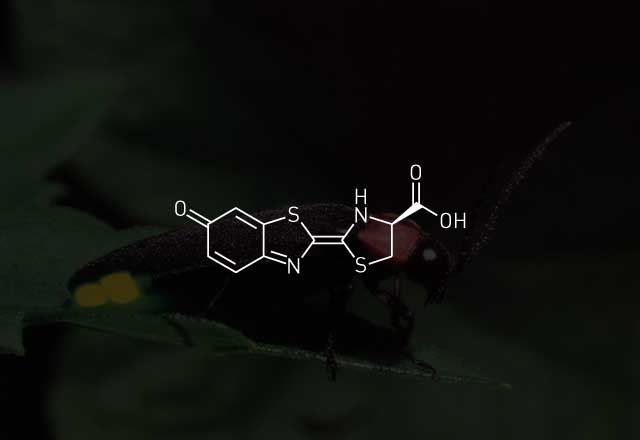
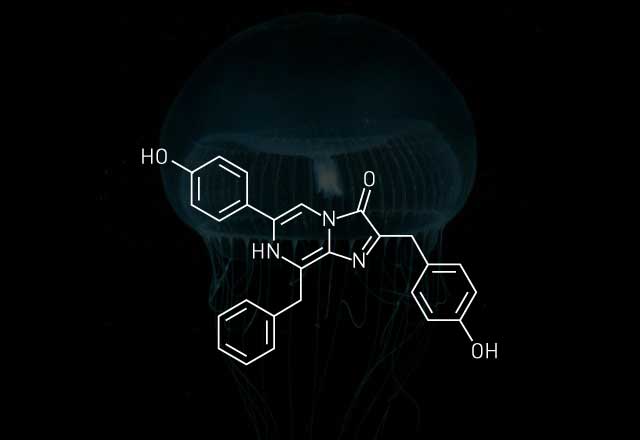
In a firefly bioluminescence reaction, an enzyme known as a luciferase uses adenosine triphosphate (ATP) to activate a molecule called a luciferin. The product of this reaction combines with molecular oxygen to produce an excited-state oxyluciferin species. When oxyluciferin relaxes back to its ground state, energy is released in the form of light.
There are variations on this theme, of course. One of the fascinating aspects of bioluminescence is how many variations have evolved. Different organisms have come up with structurally different luciferins and enzymes to attain bioluminescence. Edith A. Widder, president and senior scientist at Ocean Research & Conservation Association, in Fort Pierce, Fla., and formerly with Harbor Branch Oceanographic Institution, notes that all bioluminescence enzymes require oxygen, "but other than that, they're very, very different, and a lot of them have unique cofactor requirements."
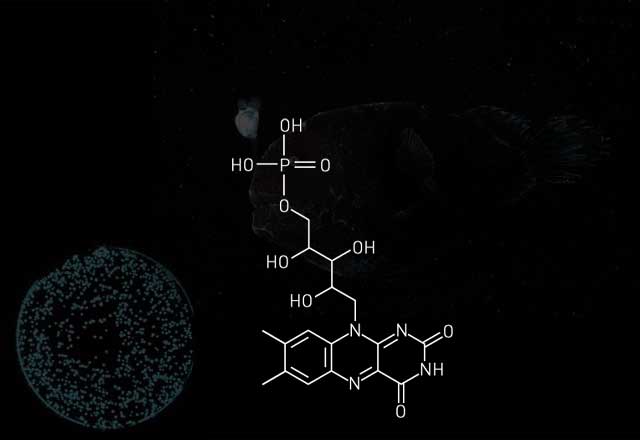
For example, fireflies require the input of energy from ATP to jump-start bioluminescence, but many marine organisms—such as jellyfish-like animals called ctenophores—can do without. Instead, they use a luciferin of intrinsically higher energy and prepackage it with oxygen in an enzyme known as a photoprotein. Calcium activates the reaction by changing the shape of the photoprotein, which releases the invested energy in the form of light.
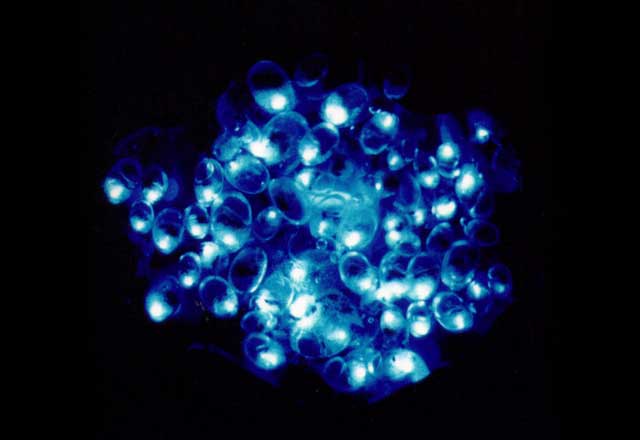
Why the need to glow in the first place? In the case of fireflies, flickering green lights are used for communication and to attract mates. Ostracods, tiny crustaceans also known as "seed shrimp" or "sea fireflies," also use bioluminescence in courtship. Males secrete luciferin and luciferase from different valves. Both mix with mucus to produce blue dots in the water, which are used to attract mates.

And if organisms aren't mating, they're eating. Steven Haddock, a marine biologist with the Monterey Bay Aquarium Research Institute, Moss Landing, Calif., recently came upon a new twist in the use of bioluminescence in the ocean. His group reported in Science (2005, 309, 263) that a siphonophore, an invertebrate animal related to a jellyfish, uses red bioluminescent lures to attract prey.
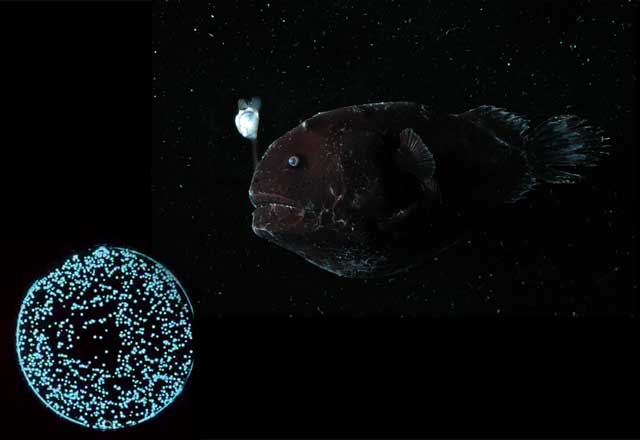
The best-known example of bioluminescent lures is the angler fish. It was unusual for Haddock to come across another species using the same rare trick. It was especially unusual that the lure is red, a color not easily visible underwater. Haddock speculates that the lures resemble copepods eaten by a particular fish. He's not sure yet what exactly causes the red bioluminescence, though he suspects there may be photoproteins within the lures. He found that rupturing a lure in a calcium solution caused a glow.
Bioluminescence and fluorescence are often lumped together, but they are not the same reaction. Both are light-emitting chemistries, and some organisms actually do use both. In a fluorescence reaction, energy is absorbed from a light source and then reemitted as a different colored light. In bioluminescence, every photon of energy is produced internally.
Each system has advantages and disadvantages in laboratory settings. For example, fluorescence is great for microscopic imaging because it emits more photons than bioluminescence, but sensitivity can be limited because it is difficult to distinguish light put into the system from light emitted by it. Because bioluminescence doesn't require the addition of photons, it can be 100-fold more sensitive than fluorescence, says Keith V. Wood, director of R&D at Promega, Madison, Wis. That sensitivity has led to some interesting applications.

One such application is in biological imaging, notes Bruce R. Branchini, a chemistry professor at Connecticut College, New London, who has studied firefly bioluminescence for more than 25 years. His group is currently working with mutant forms of luciferase to try to understand the detailed mechanism of how the amino acid residues of firefly luciferase catalyze the emission of light from its substrate. Mutants that allow for multiple colors are especially useful for in vivo imaging, he says. Red is particularly useful because it can transmit through skin better than green light.
Xenogen Corp., based in Alameda, Calif., and one of the "Glow" exhibit sponsors, has developed mouse assays that use the luciferin-luciferase reaction to trace tumor growth in live mice. Mice bearing tumors that express luciferase are injected with luciferin. Researchers then use a sensitive camera system to view, without killing the mice, the tumor and any effects of the different cancer agents.
Bioluminescence is also frequently used in assays for measuring cell proliferation, apoptosis, drug metabolism, and kinase activity. Promega's Wood points out that the simplicity and sensitivity of bioluminescence assays make them easy to automate, especially for drug screening. Wood says pharmaceutical researchers can screen the activity of more than 100,000 compounds a day using bioluminescence chemistry.
Another application involving the firefly bioluminescence reaction is pathogen detection. One example Branchini cites is the use of the firefly luciferin-luciferase reaction in food testing. Bacteria contain ATP that can kick-start the firefly bioluminescence reaction, allowing rapid and highly sensitive detection of bacterial contamination in food.
Marine-based systems also have practical applications. Wood points out that because the photoprotein aequorin requires Ca2+, it is often used to measure calcium changes inside cells.
One of the most well-known developments to come out of bioluminescence research is the discovery of the green fluorescent protein (GFP). Ocean Research & Conservation Association's Widder says "it's probably the most important discovery in the last two decades" because of GFP's impact on genetic engineering and cell biology. While GFP is not a bioluminescent protein, it serves as an accessory emitter by receiving energy from a luciferin-luciferase reaction and reemitting it as green light. Osamu Shimomura, a retired chemist from Marine Biological Laboratory, in Woods Hole, Mass., discovered the protein while he was researching the bioluminescent jellyfish Aequorea victoria.
While applications for bioluminescence continue to be developed, research is moving forward in determining more of the chemistry.

Branchini believes that researchers will learn more about the oxidative steps and color determination in bioluminescence. Already progress is being made in this area. Just last month, researchers at Kyoto University suggested how the structure of the active-site pocket that a firefly luciferase uses to bind the excited state of oxyluciferin influences the color of light emitted (C&EN, March 20, page 8).
Haddock would like to figure out where luciferins actually come from. One possibility is that organisms obtain their luciferin through diet. When working on a jellyfish kept on a luciferin-free diet, Haddock noticed that it didn't flash when stimulated. Even though the jellyfish wasn't emitting light, it was still making the proteins involved in bioluminescence. After Haddock provided luciferin, the jellyfish immediately began to glow.
Haddock also sees a return to purifying and characterizing the components of bioluminescence reactions from biological sources. "It's so much easier to just try to go in and clone the genes for a particular protein than it is to sit in a lab and extract it and characterize the actual chemicals involved," he says. "But only by doing the hard work can you figure out really novel systems."
From a practical applications standpoint, Wood anticipates rapid market adoption of bioluminescent chemistries. "I think we're going to see new assay design concepts sometime in the next year," he says. One such new concept involves engineering luciferase to incorporate molecular switches to turn the enzyme on or off as a way to track macromolecules within a cell. Promega has already been able to use protease cleavage to accomplish such a task. "It's really science fiction," Wood says. "It's amazing the things that are now happening."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter