Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
RNAi Works In Monkeys
Liposome system delivers short RNAs, gene silencing, and sustained cholesterol reduction
by Celia Henry Arnaud
March 28, 2006
| A version of this story appeared in
Volume 84, Issue 14

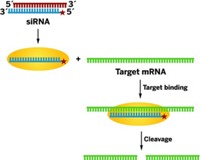
The gene-silencing technique RNA interference has moved a step closer to becoming a viable therapeutic approach. Scientists at Alnylam Pharmaceuticals in Cambridge, Mass., used RNAi in monkeys to silence the gene for apolipoprotein B (ApoB), a protein involved in cholesterol metabolism. The study was published in the online edition of Nature on Sunday (Nature, published online March 26, dx.doi.org/10.1038/nature04688). Muthiah Manoharan, vice president for drug discovery at Alnylam, also described the work on Monday in a symposium sponsored by the Division of Medicinal Chemistry at the ACS national meeting in Atlanta.
Tracy S. Zimmermann and coworkers gave monkeys a single injection of a short interfering RNA (siRNA) in a liposomal formulation optimized for delivery to the liver. Within 48 hours, expression of the ApoB gene in the liver dropped by more than 90%. The levels of ApoB, serum cholesterol, and low-density lipoproteins (commonly known as "bad" cholesterol) also dropped significantly. The effects lasted for 11 days.
In an earlier study with mice, the siRNA was conjugated to cholesterol (C&EN, Nov. 15, 2004, page 9). The liposomal delivery system was effective with doses as low as 1 mg/kg of body weight. "We're continuing to optimize the use of chemical conjugates," says John Maraganore, president and CEO of Alnylam. "At the same time, we've been exploring other ways of delivering siRNAs systemically. We believe that multiple technologies can be brought to bear."
John J. Rossi, a molecular biologist who studies RNAi at the Beckman Research Institute of the City of Hope in Duarte, Calif., cites as an important advance the fact that a lower dose is needed with the current delivery system than with the cholesterol conjugate. "The amount of material that's going to be needed to get a systemic effect is much less than shown previously with the cholesterol-tagged molecule," he says.
Although Alnylam has yet to declare its first candidate for systemic RNAi, the company continues to move toward clinical trials. "With liposomal technologies, we have a near-term opportunity and solution that can be used to begin to build our clinical pipeline," Maraganore says. "We're now in the position where we can begin to advance systemic RNAi therapeutics toward human clinical testing as early as the next 18 to 24 months."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter