Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Ketenes Turn 100
Chemists gather to celebrate centennial of synthetically useful family of compounds
by Amanda Yarnell
January 9, 2006
| A version of this story appeared in
Volume 84, Issue 2

In 1905, Hermann Staudinger, a young instructor at Kaiser-Wilhelms University in Strasbourg on the German-French border, managed to isolate the first example of what is now a large, synthetically valuable functional class, the ketenes. At last month's International Chemical Congress of Pacific Basin Societies (Pacifichem), held in Honolulu, chemists gathered to honor ketenes' 100th birthday and to discuss some of the latest advances in ketene chemistry.
Despite Staudinger's success in isolating diphenylketene, "most ketenes are intrinsically unstable and resist isolation," noted Rick L. Danheiser, a synthetic organic chemist at Massachusetts Institute of Technology who co-organized the centennial celebration symposium with physical organic chemist Thomas T. Tidwell of the University of Toronto. The prototypical ketene, H2C=C=O, is reactive and can be isolated only at -80 oC. Like other ketenes-even those stable enough to be isolated-nucleophiles readily add to its carbonyl, while its C=C bond readily undergoes cycloaddition reactions.
Ketenes' reactivity has earned them an enduring place in the synthetic chemist's toolbox. Research presented at Pacifichem reflected not only ketenes' past but also their future. Today, chemists are using ketenes to make highly substituted chiral cyclic compounds in natural product syntheses. In addition, researchers continue to strive to make ketene chemistry more broadly useful by easing the generation of these reactive compounds.
Staudinger himself was the first to demonstrate that ketenes provided easy access to functionalized four-membered rings that are otherwise difficult to make. He proved the value of ketenes by reacting them with alkenes to make cyclobutanones, by reacting them with imines to make β-lactams, and by reacting them with carbonyl compounds to make β-lactones, Tidwell noted in a review (Angew. Chem. Int. Ed. 2005, 44, 5778). These cycloaddition reactions "are still mainstays of synthetic organic chemistry and today remain the most useful aspect of ketene chemistry," Tidwell told C&EN. Their mechanisms were reported by Takahisa Machiguchi of Saitama University in Japan and David Birney of Texas Tech University.
Staudinger prepared the first ketenes by dehalogenating α-chloro acetyl chlorides. Other methods soon emerged, notably the preparation of H2C=C=O by thermolysis of acetone with a hot platinum wire, pioneered by N. J. M. Wilsmore of University College, London, in 1907 and perfected by Charles D. Hurd of Northwestern University in 1940. Hurd's apparatus for ketene production-commonly known as a Hurd lamp-is still used in many synthetic labs, including Danheiser's. It's also used in the industrial preparation of ketene for conversion into acetic anhydride.
More recently, researchers have begun to look for ways to generate ketenes in situ to encourage their broader use, according to Daniel Romo of Texas A&M University. He and others now use a practical procedure for generating ketene in situ via the dehydrohalogenation of commercially available acyl chlorides, a route pioneered by Scott Nelson of the University of Pittsburgh. Mitsuru Shindo of Kyushu University also described an alternative, ynolate-mediated method of ketene generation.
As reflected in the program at Pacifichem, much of the current synthetic interest in ketene chemistry is focused on using it to generate new stereogenic centers, a pursuit first championed by Horst Pracejus of the University of Rostock in the 1960s. Pracejus used chiral base catalysts to make chiral esters and chiral amides from aryl(alkyl)ketenes.
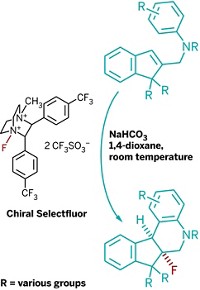
Michael A. Calter of Wesleyan University showed that his lab has used amine bases to catalyze asymmetric ketene cycloadditions leading to chiral β-lactones and β-lactams. These reactions build on work published by Dutch chemist Hans Wynberg in 1982, Calter noted. Nelson described how his lab has used similar chemistry to stereoselectively synthesize an inhibitor of microtubule assembly boasting antitumor activity. And Romo reported intramolecular ketene-aldehyde cycloadditions that yield interesting chiral carbocycle-fused β-lactones.
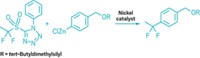
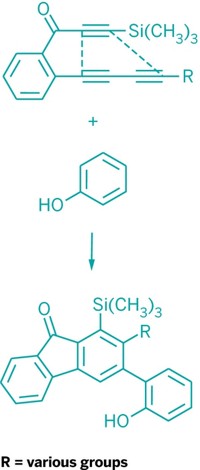
William D. Wulff of Michigan State University described how his lab combines Fischer carbene complexes and alkynes to generate a doubly unsaturated ketene that then cyclizes to give alkoxyphenols and chiral cyclohexadienones. Wulff's team has used this reaction in the total syntheses of plant cannabinoids having antimalarial activity, a natural product involved in clotting, and unsymmetrical calixarene macrocycles. Danheiser described how vinylketenes and other conjugated ketenes can be used to construct highly substituted carbocyclic and heterocyclic ring systems.
These and other synthetic uses for ketenes will appear in an upcoming 800-page volume on ketene chemistry to be published by Thieme this winter, noted Danheiser, who edited the volume. A second edition of Tidwell's book "Ketenes" will be published soon by Wiley.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter