Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Second known Fe(VI) complex debuts
June 5, 2006
| A version of this story appeared in
Volume 84, Issue 23
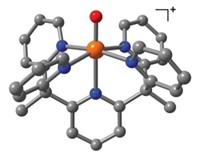
Chemists have been on the lookout for high-oxidation-state iron complexes with metal-ligand multiple bonds because they are proposed intermediates in biocatalytic pathways, such as the enzymatic reduction of N2 and O2. Though several Fe(IV) and Fe(V) complexes have been prepared, only one Fe(VI) complex was known before now: the ferrate anion, FeO42-, which serves as a useful oxidizing agent in several applications. John F. Berry and Karl Wieghardt at the Max Planck Institute for Bioinorganic Chemistry, in M?lheim an der Ruhr, Germany, and their colleagues now report the second Fe(VI) compound in the form of the iron nitride shown (Science, published online June 1, dx.doi.org/10.1126/sci ence.1128506). The complex was prepared from an Fe(III) azide complex, FeN3L, where L is the trimethylcyclam acetate ligand. The Fe(III) complex was electrochemically oxidized to Fe(IV), then photochemically oxidized at 77 K to cleave N2 and form the Fe(VI) complex, which remains stable at low temperature. Coupled with other recent advances, "these results portend both a much greater oxidation-state flexibility and multiple-bond chemistry for iron," notes MIT chemist Christopher C. Cummins.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter