Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Triple Cascade
Asymmetric reaction constructs three new C-C bonds and four stereocenters
by Bethany Halford
June 19, 2006
| A version of this story appeared in
Volume 84, Issue 25

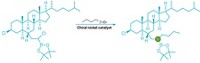
Some of the most elegant reactions in organic synthesis work just like an elaborate arrangement of dominos: Set up the reagents just so, and all it takes is a little catalytic push to make the pieces tumble into a far more elaborate whole. Such is the case with an ingenious new cascade reaction wherein a simple organic catalyst is the key element in the construction of a complex tetrasubstituted cyclohexene carbaldehyde (Nature 2006, 441, 861).
The three-step pathway, developed by Dieter Enders, Matthias R. M. Hüttl, and Christoph Grondal of Germany's RWTH Aachen University, was inspired by the tandem reactions that nature uses to biosynthesize complex natural products. The sequence employs two Michael-type reactions and an aldol condensation, ultimately creating three new C-C bonds and establishing four stereocenters with "high diastereoselectivity and complete enantioselectivity," according to the researchers.

"This is an outstanding example of simple organic chemistry achieving very sophisticated results, results reminiscent of enzymatic finesse," remarks Johns Hopkins University chemistry professor Gary H. Posner.
To catalyze this carefully choreographed sequence, the Aachen group chose a simple secondary amine derived from the amino acid proline. Organocatalysts of this kind are emerging as a powerful tool in asymmetric synthesis. The small molecules are usually nontoxic and robust as well as inexpensive because they are often derived from readily available chiral compounds.
Enders, Hüttl, and Grondal think the organocatalyst plays a crucial role in all three steps of the cascade. In the first step, they believe it activates a linear aldehyde via enamine formation so that it selectively adds to a nitroalkene. Upon hydrolysis, the catalyst then forms an iminium ion with an α,β-unsaturated aldehyde, which undergoes conjugate addition with the compound formed in the first step. Finally, further catalytic enamine activation initiates an intramolecular aldol condensation to close the six-membered ring.
Although the reaction could generate 16 different stereoisomers, just two diastereomers are formed. "The reason for the high stereoselectivity is the first Michael addition, which is known to proceed with high diastereo- and enantioselectivity," the researchers explain. The resulting product presumably dictates the stereochemistry of the subsequent reactions.

"This work is a remarkable example of modern approaches for designing catalytic methods that perform multiple concurrent transformations with excellent stereoselectivity in each step," says Guillermo C. Bazan, a professor of chemistry and materials at the University of California, Santa Barbara. "The strategy is deceptively simple: Pick three readily available achiral starting materials, add a simple organocatalyst, and collect a product with four stereogenic centers. That the authors have been able to demonstrate three consecutive reactions in a single procedure shows remarkable creativity."
"I think this is the future of organic chemistry," Enders tells C&EN, "building molecules as Mother Nature does."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter