Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalyst In A Cage
Researchers improve oxidation catalysis by incorporating ligands into metal-organic framework
by Rebecca Evanhoe
June 26, 2006
| A version of this story appeared in
Volume 84, Issue 26

Conventional homogeneous and heterogeneous catalysts each have their advantages and disadvantages. While heterogeneous catalysts (those in a different phase from the reactants, such as a solid catalyst in a liquid) are robust and recyclable, they often require high temperatures to function and few can facilitate asymmetric reactions. On the other hand, many homogeneous catalysts (those in the same phase as the reactants) can promote enantioselective reactions and work at low temperatures, but they aren't easily reusable.
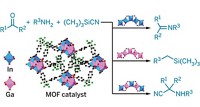
Now a team of researchers at Northwestern University and at Auburn University, in Alabama, have unveiled a metal-organic framework (MOF) compound that is one step closer to combining the best of both worlds for olefin epoxidation reactions. By taking a known catalyst and locking it into an MOF structure, the team has managed to make a new oxidation catalyst that overcomes some earlier limitations. Mimicking most zeolitic catalysts, the new catalyst is reusable, stable, and size-selective. In addition, the framework material, which catalyzes olefin epoxidation, produces a chiral product, a feature more often attributed to homogeneous catalysts (Chem. Commun. 2006, 2563).
"This is among the first attempts to combine homogeneous catalysts in a metal-organic framework to make them more stable and recyclable, and at the same time maintain the advantages of the homogeneous catalyst," says SonBinh T. Nguyen, a professor of chemistry at Northwestern. Nguyen led the study with his Northwestern colleague Joseph T. Hupp, along with graduate student So-Hye Cho, postdoctoral fellow Baoqing Ma, and Thomas E. Albrecht-Schmitt, an associate professor of chemistry at Auburn.
To make the catalyst, the team heated a mixture of zinc nitrate, biphenyldicarboxylic acid, dimethylformamide, and a dipyridyl manganese salen ligand [(salen)Mn], a version of the well-known Jacobsen-Katsuki homogeneous epoxidation catalyst. After more than a week, brown block-shaped crystals formed. When analyzed by X-ray powder diffraction, the crystals revealed an organic framework with (salen)Mn catalysts serving as "pillars" in the microporous structure.
This unit cell can be stacked infinitely in all three dimensions, "akin to stacking bricks together to build up a cube of bricks," explained Nguyen.
The product is a material with big pores, like a chain-link fence, in which each opening serves as a passageway for molecules. In this way, the MOF material functions like a chiral zeolitic catalyst: It selectively produces the desired molecule in 82% enantiomeric excess-comparable to using the soluble form of the (salen)Mn catalyst, which yields 88% ee.

Another useful feature is that the catalyst can be used for several trials without significantly reducing its catalytic activity. Hupp and Nguyen's team has tested the catalyst in three separate runs with minimal loss in activity. The team reports that the yield dropped 5% after the first two runs.
The extended lifetime results from immobilizing the catalyst within the framework material. As Nguyen explains, in homogeneous reactions, "free-range" (salen)Mn ligands floating in solution encounter other catalytic molecules and decompose, either through oxidation or hydrolysis, thus eliminating their catalytic activity. "The ligands just fall apart," Nguyen remarks. But locking the salen ligand into place keeps it out of contact with other catalysts that could cause it to decompose, leaving the molecule and its catalytic powers intact.
Experts say the catalyst epitomizes the potential of MOF materials for future catalyst development. "Metal-organic frameworks are the realization of a long-standing 'dream' for chemists," says Omar M. Yaghi, a chemistry professor at the University of California, Los Angeles, who is a pioneer in the design and applications of MOFs. "This report speaks volumes to the usefulness of the MOF design approach in catalysis."
The next goal of the team, according to Hupp and Nguyen, is to facilitate other reactions by replacing the catalytic struts with copper or iron complexes so that the framework might use oxygen as the oxidation reagent.
The team also hopes to couple the catalyst to a membrane, so that reactions can be run by passing the reagent through a catalyst-lined column or film.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter