Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Fluorous Phase In A Cage
Self-assembling nanoshell sequesters perfluoroalkanes with exquisite control
by Bethany Halford
September 4, 2006
| A version of this story appeared in
Volume 84, Issue 36

Most chemists would consider a 5-mL round-bottom flask an appropriate vessel for a small-scale reaction. But for the researchers in Makoto Fujita's lab, that's about a million times too big.
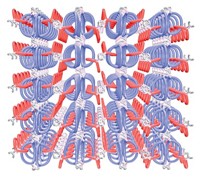
Fujita, a chemistry professor at the University of Tokyo, and his colleagues have been making nanoscale reaction chambers just 5 nm wide. Their latest invention, a tiny cage lined with 24 perfluorinated carbon chains, offers a finely tuned environment for fluorous chemistry (Science 2006, 313, 1273).
There are many other examples of fluorous microenvironments, notes chemistry professor John A. Gladysz of Friedrich-Alexander University, Erlangen, Germany, in a commentary on the report. "However, none of these assemblies features the elegant design elements and the degree of molecular control that characterize" Fujita's complexes.
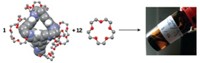
Fujita's team designed an arrow-shaped molecule as their structure's basic building block. A bridging ligand forms the arrowhead, which attaches to a long fluorocarbon tail. In solution, 24 of these arrows coordinate 12 palladium ions and spontaneously assemble into a nanocage.
The fluorocarbon chains reach into the structure's core like a dendrimer in reverse. X-ray crystallography data indicate that these chains are disordered, essentially forming a nanosized droplet of fluorous liquid. Perfluoroalkanes that are only sparingly soluble in polar organic solvents easily dissolve within the structure, creating a nanosized reaction chamber. Each nanoshell can encapsulate up to six perfluorooctane molecules, depending on the length of the fluorocarbon tail.
Dennis P. Curran, of the University of Pittsburgh, says Fujita's "elegantly designed and painstakingly crafted molecular spheres" could easily win a "Frank Lloyd Wright Award for Creativity in Molecular Architecture", if such an award existed.
Curran sees both beauty and strength in the structure's exterior. "There's no door, but there are plenty of windows, and the residents seem quite content to enter and exit this way," he explains. "The sphere is furnished almost entirely with fluorines. If you are a fluorous molecule, the interior space is so warm, inviting, and ergonomic that you would much rather be inside than out."
Fujita would like to use the fluorine-friendly interior to perform chemical reactions that work well in the fluorous phase. Developing selective oxidations is particularly attractive. Small molecules such as methane and oxygen could slip into the nanoshell's fluorous interior and react. The resulting methanol would escape from the cage before being further oxidized.
The Tokyo group is also working to make nonfluorous nanoflasks for other types of chemistry. "Contributions to organic synthesis, materials science, and biology will be our next challenge," Fujita tells C&EN. "By encapsulating photopolymerizing monomers, we expect the construction of high-density data-storage materials. Encapsulation of biomolecules, particularly proteins, could lead to the control of biofunctions."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter