Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Antimalarial Agent Hits Novel Enzyme
Identification of a fresh target in malaria parasite could lead to new class of drugs
by Stu Borman
September 21, 2006
| A version of this story appeared in
Volume 84, Issue 39

Scientists have found a compound that potently inhibits a novel target protein in the malaria parasite, including forms of the parasite resistant to the classic antimalarial drug chloroquine. The compound could portend a new class of agents to fight malaria.
Malaria, an infection caused by the protozoan Plasmodium falciparum and transmitted by mosquitoes, causes high fever, body aches, and often death, particularly among children. Despite decades of effort to eradicate the disease, it still kills more than 1 million people per year, mostly in Africa. Current medications can't adequately address the problems posed by malaria worldwide, and new treatments that are more effective, cheaper, and easier to distribute are urgently needed.
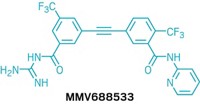
One problem is that few targets for antimalarial drugs are known. Now, pharmacology professor Jun O. Liu of Johns Hopkins University School of Medicine and coworkers have identified a methionine aminopeptidase (MetAP) enzyme from P. falciparum as a new molecular target for malaria treatment. And by screening a 175,000-compound library, they were able to find a lead compound called XC11 that inhibits the enzyme with good potency (Proc. Natl. Acad. Sci. USA 2006, 103, 14548). Liu also collaborated with associate professor of molecular biology and immunology David J. Sullivan Jr. of Johns Hopkins Bloomberg School of Public Health in the recent discovery of another promising antimalarial agent, astemizole (Nat. Chem. Biol. 2006, 2, 415; C&EN Online Latest News, July 5).
XC11 selectively inhibits MetAP 1b without blocking the activity of the other three MetAPs encoded in the P. falciparum genome. The compound shows good activity against both chloroquine-sensitive and chloroquine-resistant strains of the malaria parasite in a mouse model. Improving the selectivity and in vivo potency of XC11 analogs, efforts the researchers are currently pursuing, "may lead to the development of a novel class of antimalarial agents," they note in their paper.
Peter K. Chiang, chief scientific officer of Pharmadyn, in Sunnyvale, Calif., says that the new study is proof of concept of earlier observations by his group that inhibiting MetAP enzymes has antimalarial and antiparasitic effects. The Johns Hopkins study "is an important milestone discovery pointing to a new paradigm in using MetAP inhibitors as novel antimalarial agents to circumvent malarial drug resistance," Chiang says.
Chemistry professor David H. Peyton of Portland State University, in Oregon, a specialist in antimalarial drug design, says, "It is to be hoped that this lead compound will yield a productive search for a class of drugs that might help to alleviate the disaster that is found through much of the developing world???drug-resistant strains of malaria."
MetAPs are protease enzymes, and "there are currently no protease-directed antimalarial drugs in use," says assistant professor of pathology Matthew S. Bogyo of Stanford University School of Medicine, "so compounds like XC11 are likely to be active against the current resistant strains of the parasite." Bogyo notes that it will be interesting to compare XC11 and related agents with another set of protease inhibitors that are being developed by professor of medicine Philip Rosenthal of the University of California, San Francisco, in collaboration with GlaxoSmithKline through the Medicines for Malaria venture, a cooperative public-private effort to discover, develop, and distribute new antimalarial drugs.
Rosenthal, whose group is investigating inhibitors of cysteine proteases, notes that the key advance in the new study is not that XC11 is a protease inhibitor per se but that the Johns Hopkins group found a new antimalarial target, of which there are currently very few, and used high-throughput screening to identify a lead compound that hits it.
"It's still relatively early in the pathway to drug discovery and development," Rosenthal says of the new study. "XC11 has a potency a little better than 100 nM against the enzyme and a bit worse against the parasite. That's pretty good, but there is a long way to go." Nevertheless, Liu and coworkers "have accomplished a lot in this one paper, and it's an excellent first step."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter