Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Targeting Telomerase
Researchers believe enzyme could be a nearly universal target for anticancer drugs
by Stu Borman
October 9, 2006
| A version of this story appeared in
Volume 84, Issue 41
Telomerase may be a nearly universal anticancer drug target: Inhibitors of this human enzyme might be able to treat most cancers without significant side effects.
That theory hasn't been verified yet clinically, but it was nevertheless the overarching theme of a Division of Medicinal Chemistry session on telomerase at last month's American Chemical Society national meeting in San Francisco. The session was organized by assistant professor of medicinal chemistry Brian S. J. Blagg of the University of Kansas, Lawrence, and assistant professor of medicinal chemistry and natural products Michael B. Jarstfer of the University of North Carolina School of Pharmacy, Chapel Hill. Speakers described their efforts to design inhibitors of telomerase and to better understand the structure and function of the enzyme and its target.
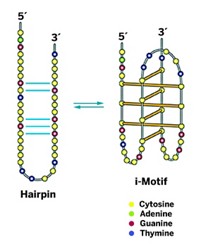
Telomerase is an enzyme that catalyzes the lengthening of telomeres, DNA structures found at the ends of chromosomes. Telomeres are mostly double-stranded DNAs with a repeating TTAGGG sequence on one strand and a complementary sequence on the matching strand. With all that repetitiveness, you might expect them to be boring. But they're really not, because this repetitive part of the chromosome just might hold the key to treating cancer more effectively.
The potential role of telomeres as near-universal anticancer targets stems from their tendency to shrink over time. When cells divide by mitosis, their chromosomes are replicated, and telomeres get shorter with each replication. This shortening occurs because the DNA polymerases that catalyze replication can't copy the very end of each telomere, making new telomeres 50 to 100 base pairs shorter each time chromosomes are copied. When telomeres get critically short, cells stop dividing and commit suicide.
Cancer cells divide more than normal somatic cells. If their telomeres were to shorten with each mitotic event and not be restored, they might have to commit suicide, too, which would crimp their propensity to proliferate and take over tissues.
So most cancer cells have developed a vampirelike means of maintaining their telomeres. To live forever, so to speak, they express telomerase, an enzyme that catalyzes replacement of the telomeric region lost during replication.
Telomerase has both an RNA subunit, which serves as a template for telomere lengthening, and a catalytic protein subunit, which catalyzes the extension reaction. Telomerase is expressed only in cells that proliferate a lot, such as embryonic cells, germline cells, and some stem cells, in addition to cancer cells. It's not usually expressed in nonproliferative normal somatic cells.
This is a potentially useful distinction. "Since telomere maintenance is one of the absolute requirements for a cancer cell to continue dividing, it makes sense that if you knock out telomere maintenance, which most cells don't have to worry about because they're not dividing frequently, then cancer cells won't be able to proliferate," Jarstfer said. So if you use a drug to inhibit telomerase, you'll probably attack the cancer and perhaps see side effects in some other proliferative cells, but you're not likely to see adverse effects in most body cells because they don't express telomerase. Voilà: selective anticancer activity.
Telomerase can be targeted in vivo via various means, such as small organic molecules, oligonucleotide-based compounds, or immunotherapeutic agents. Speakers at the meeting session discussed some of these approaches.
For example, associate professor of pharmaceutical sciences Simon H. Friedman and coworkers at the University of Missouri, Kansas City, are trying to inhibit telomerase by using small molecules to target the RNA-DNA duplex that forms between telomerase's RNA template strand and the telomere's terminal DNA strand at the start of the telomere extension process.
Friedman and coworkers believe that small molecules might inhibit telomerase either by distorting the duplex, which would make addition of new bases difficult, or by overstabilizing the duplex, which would hinder unwinding, an essential step in the DNA replication process. They also hope the studies will help them identify key interactions between the small molecules and surrounding telomerase surfaces.
He and his coworkers are currently making large libraries of small molecules that can be screened for inhibitory activity. "We're aiming for a low- or subnanomolar inhibitor, and we haven't gotten there yet," Friedman said.
Jarstfer and coworkers also want to inhibit telomerase, but they're trying to do so with oligonucleotides instead of with small organic molecules. Their strategy is to use oligonucleotides to block specific RNA-protein contacts that are required for the protein and RNA components of telomerase to assemble properly in cells. Oligonucleotides interfere with correct assembly of the telomerase complex, making the complex incapable of catalyzing telomere extension in vitro.
One oligonucleotide analog is already on the way to perhaps becoming the first approved telomerase inhibitor for treatment of cancer. Called GRN163L, it is currently in human clinical trials sponsored by Geron, Menlo Park, Calif. GRN163L is a lipid-conjugated thiophosphoramidate. Its nucleotide sequence is complementary to that of telomerase's RNA template, enabling it to bind the telomerase active site and thus inhibit the enzyme.
Geron Director and Senior Research Fellow Sergei M. Gryaznov and coworkers discovered GRN163L by synthesizing phosphoramidates and thiophosphoramidates and identifying some that inhibited telomerase fairly effectively. They optimized the compounds' sequence and length, and they enhanced the analogs' ability to be absorbed by cells by conjugating them with lipophilic groups.
Gryaznov and coworkers identified GRN163L as one of their best inhibitory compounds. When they treated cancer cells with GRN163L, the agent caused telomere shortening and cell death without affecting the growth of normal cells. It also showed potent antitumor activity in animal models. Geron therefore selected it as a development candidate, and it is currently in Phase I and I/II clinical trials for patients with solid tumors and chronic lymphocytic leukemia, respectively.
Telomerase researchers are also studying immunotherapeutic agents, which stimulate an immune response against cancer cells that express telomerase. "Cancer cells present telomerase peptide fragments on their cell surfaces, where they act as antigens," Jarstfer explained, but healthy somatic cells, germline cells, and stem cells do not do this. Telomerase-based immunotherapeutics can thus immunize people against cancer cells by inducing their bodies to produce immune cells that can kill the cancer. One such vaccine developed by Geron in collaboration with Duke University has completed Phase I/II trials in prostate cancer patients, and an immunotherapeutic developed by Pharmexa, in Hørsholm, Denmark, is going into Phase II clinical trials.
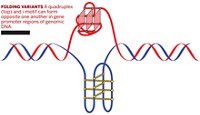
When trying to identify agents that inhibit telomerase activity selectively, it's important to consider the structural and functional aspects of the telomere, reported associate professor of medicinal chemistry Sean M. Kerwin of the University of Texas, Austin. Telomere ends are known to be able to adopt four-stranded conformations called G-quadruplexes in cells, and some anticancer agents are being designed to bind to G-quadruplexes. Tying up the telomere's G-quadruplex could prevent telomerase from functioning properly and thus inhibit telomere lengthening.
But the telomere sequence is just one of 300,000 or so sequences in the human genome that have been predicted to be capable of folding into G-quadruplexes. "About three years ago we started worrying about other effects G-quadruplex-interactive agents might have as a result of interacting with nontelomeric G-quadruplexes or by interfering with other enzymes besides telomerase that interact with G-quadruplex DNA," Kerwin said.
For instance, some telomerase inhibitors also inhibit a helicase enzyme called SV40 T-antigen. This prevents T-antigen from unwinding G-quadruplex DNA during replication. Therefore, "these telomerase inhibitors might display nonspecific effects on DNA replication in both cancer and normal cells, leading to toxicity," Kerwin said. "We were able to identify a few G-quadruplex-interactive agents that selectively inhibited telomerase and not T-antigen." He suggested that these would tend to be less toxic than nonselective quadruplex-interactive agents. His group's work thus has implications "for understanding the way in which different G-quadruplex-associated proteins recognize these fascinating DNA structures and how small molecules can be designed to interfere with this recognition in a very selective fashion," Kerwin said.
Researchers are also trying to better understand the structural aspects of telomere folding. For example, assistant professor of pharmacology and toxicology Danzhou Yang of the University of Arizona, Tucson, and coworkers recently were one of two groups that determined, using nuclear magnetic resonance spectrometry, a solution structure of the G-quadruplex formed by human telomeres in potassium solution (Nucleic Acids Res. 2006, 34, 2723; C&EN, July 31, page 46).
"Our results demonstrated a novel, unprecedented intramolecular G-quadruplex folding topology," Yang said. The distinct folding topology of the structure suggests that it might be possible to target it with small-molecule drugs in a highly selective manner.
What drug designers would really like to know is exactly how telomerase interacts with telomeric G-quadruplexes. Holding back such studies is that "we don't have a structure of telomerase," although partial structures have been obtained, Jarstfer said. "The main problem is obtaining large quantities of the purified [RNA-protein] complex."
Whether telomerase turns out to be a near-universal anticancer target remains to be seen. But with the enzyme being targeted and studies progressing in so many different ways, researchers are quickly closing in on a deeper understanding of how it works and how it can be controlled therapeutically.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter