Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Laser Guides Reaction Outcome
Novel method uses light's electric field to steer reactions toward selected products
by Mitch Jacoby
October 16, 2006
| A version of this story appeared in
Volume 84, Issue 42

The outcome of photochemical reactions can be selected by a new laser method that's based on an effect discovered nearly a century ago. The study broadens understanding of interactions between light and matter and may provide a means for controlling quantum phenomena in various applications.

Using laser light to steer chemical reactions toward specific products, especially ones that are not easily formed by controlling reaction temperature or pressure or by other conventional means, has been a goal of researchers for years. One approach to the problem calls for initiating a reaction with a low-energy laser pulse that nudges the reactants in the direction of the desired products, then stepping back and letting the process unfold. The method is akin to giving skiers at the top of a mountain a gentle starting push that points them toward one of several valleys below. The landscape represents the potential energy surface.
Another way to direct the process is to zap the molecules with intense pulses that force the reactants toward selected ionic products. That tack, studied by some research groups, is like shoving the skiers off one mountain (a neutral potential energy surface) and onto another mountain (an ionic surface).
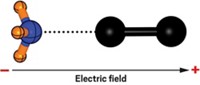
Now, scientists at the Canadian National Research Council (CNRC), in Ottawa, have developed a method that avoids forming ions, which may not always be the desired products, yet exerts fine control over the reactants. The new technique makes use of a precisely timed pulse of near-infrared light that functions as a catalyst by reversibly modifying potential energy barriers during the course of a reaction. The method was demonstrated with a simple test case—dissociation of IBr—by guiding the reaction toward one of two pairs of products: I and Br (neutral) or I and Br* (excited) (Science 2006, 314, 278).
Albert Stolow, who led the study at CNRC's Steacie Institute for Molecular Sciences, explains that in terms of the ski slope analogy, the new method temporarily modifies the contours and landscape of the slopes while the skiers are traversing the mountain. By creating a short-lived downhill path that leads to the desired end point just as the skiers are approaching, the method guides the skiers toward the chosen outcome on the fly. The mountain reverts to its original landscape a moment later.
Unlike most laser-based procedures, the new method, developed by Stolow, Benjamin J. Sussman, Dave Townsend, and Misha Yu Ivanov, does not depend on the frequency of the radiation, Stolow points out. Instead, it's the electric field associated with the IR pulse that briefly alters the molecule's energy levels. That process is the dynamic version of the classic Stark effect, which is a shift in a molecule's energy levels under the influence of an electric field. Another key feature of the technique is that no light is absorbed by the reactants. That distinction precludes the need to tune the light to the sample's resonant frequencies and may make it applicable to many types of samples.
In a commentary in the same issue of Science, Princeton University chemistry professor Herschel A. Rabitz describes the method as a "clear experimental illustration" of energy landscape manipulation and notes that the procedure may be extended to polyatomic molecules and complex systems.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter