Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Breath Test
No-prep method analyzes nonvolatile components in breath
by Celia Henry Arnaud
November 20, 2006
| A version of this story appeared in
Volume 84, Issue 47

Breath analysis usually measures just the volatile components of breath. Swiss scientists now show that mass spectrometry can be used to measure semivolatile and even nonvolatile compounds in breath. Such a method could be used for metabolic studies or diagnostic tests.
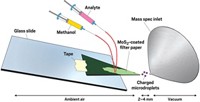
Renato Zenobi and coworkers at the Swiss Federal Institute of Technology, Zurich, use an unmodified electrospray ionization source to deliver the breath straight into the mass spectrometer (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200602942). Instead of mixing the sample with solvent first, they use the electrospray source's sheath-gas line—which usually assists in breaking up solvent droplets—to bring the breath to the charged solvent. The electrical charge is transferred directly from the solvent to the analytes in breath. The method, called extractive electrospray ionization (EESI), is a variant of desorption electrospray ionization (DESI).
Zenobi was surprised at what they could see. "We thought with breath you would just see the volatile compounds, but breath is in fact an aerosol," he says. "We think that some of the small droplets in the breath carry sugars and other nonvolatiles." For example, they were able to detect urea. They also measured nicotine and its metabolites in a female smoker's breath and compared alcohol metabolism in Asian and European males.
They simulated bad breath by eating garlic and then breathing into the instrument. At first, they had difficulty detecting the thiol compounds that give garlic its characteristic odor. But by using silver nitrate in the electrospray solvent, they were able to ionize the thiols. The silver converts the sulfur-containing molecules to cations "very easily," Zenobi explains, "so you see them right away."
"This study is interesting because the sample "breath' has been much studied by MS for its volatile constituents but not for its nonvolatiles, which presumably carry more biological information," says R. Graham Cooks of Purdue University, the original inventor of both DESI and EESI.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter