Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Crystal Ball On The Environment
Detective work and expertise are used to evaluate environmental contaminants of emerging concern
by Stephen K. Ritter
January 30, 2006
| A version of this story appeared in
Volume 84, Issue 5

If chemists could easily predict which new chemicals would lead to environmental health and safety problems, they could forestall a lot of problems. Chemicals sometimes end up being used for years before they are found to be contributing to an environmental problem; polychlorinated biphenyls (PCBs) and chlorofluorocarbons are just a couple of examples.
Today, armed with hindsight, ever-better analytical techniques, and the tenets of green chemistry, chemists are starting to ferret out troublemaking chemicals early on, before problems get out of hand. And once manufacturers or regulatory agencies get an inkling that a class of compounds potentially could become an environmental concern, they can work together to solve the problem. Perchlorate, polybrominated flame retardants, and long-chain perfluoroalkyl compounds are a few current examples.
Discussions involving these concepts and descriptions of environmental studies in progress were the highlights of a symposium on environmental pollutants of emerging concern held last month at the 5th International Chemical Congress of Pacific Basin Societies (Pacifichem) in Hawaii.
To qualify as a contaminant of emerging concern, a compound must meet three criteria, according to environmental chemist A. Lynn Roberts of Johns Hopkins University. Appreciable amounts of the compound must be entering or be generated in the environment, the compound must have "a modicum of persistence," and it must exhibit deleterious effects on organisms, Roberts said. Compounds that satisfy these criteria, if not already regulated or considered priority pollutants, should be candidates for environmental monitoring, she noted.
Roberts went on to describe her group's research on monitoring the degradation products of chloroacetamide herbicides. Acetochlor, metolachlor, and other compounds in this class are some of the most widely used agricultural chemicals. In sampling part of the Chesapeake Bay, graduate student Michelle L. Hladik in Roberts' group found that the total concentration of 19 chloroacetamide degradation products, each detected in the parts-per-trillion range, was 20 to 30 times greater than that of the parent compounds (Environ. Sci. Technol. 2005, 39, 6561).
Many of these degradates satisfy the emerging contaminants criteria, Roberts said, and they need to be studied further to fully assess the potential impact of chloroacetamides on the environment. But the degradates haven't yet received much attention in the U.S. because regulating degradates is a relatively new practice and there is a lack of commercially available reference standards and validated test methods for their analysis, she pointed out.
One reason for that shortcoming is simply that the Environmental Protection Agency doesn't have the time or the resources to collect or measure all of the chemical property and toxicity data, commented Eric J. Weber, acting director of EPA's Ecosystems Research Division of the National Exposure Research Laboratory in Athens, Ga. Some 60,000 chemicals need to be assessed, and that list is growing, Weber noted. "EPA is losing the battle," he said. "We are getting further and further behind."
Computational chemistry is one way to fight back and narrow the list down to a manageable level, Weber said. He discussed a "major new EPA program" to develop computational toxicology software that can use measured and computed data to drive environmental fate and toxicity pathway simulations based on functional group reactivity and overall chemical structure. The software should help EPA over the next decade "to prioritize and test chemicals that really need testing and identify chemicals of emerging concern," he said.
Civil and environmental engineering professor David L. Sedlak of the University of California, Berkeley, echoed the views of Roberts and Weber. "It's relatively easy for researchers to ask questions about new contaminants, but it's difficult to assess the overall significance of the problems," Sedlak commented.
Municipal wastewater is probably the biggest concern. Even after being treated, the water still contains very low concentrations of dozens of contaminants of concern, principally human drug metabolites and animal steroid hormones and antibiotics, Sedlak said. Complicating matters is that chlorine or chloramines used for wastewater treatment can actually convert contaminants into more problematic compounds. Gaining a better understanding of the nature of these contaminants is becoming critical, he noted, because wastewater effluent flowing into rivers is starting to become an important source of potable water in arid regions.
To address this need, Sedlak's group is developing a suite of in situ chemical indicators—contaminants that are already in the water—that can be used to analyze water-supply systems and aquatic ecosystems. Application of these analytical tools could provide a better understanding of how wastewater-derived contaminants are eliminated in treatment systems, in the environment, and in engineered wetlands designed to remove contaminants, Sedlak said.
For example, untreated sewage often gets into waterways from leaking sewer lines or from overflows of "combined" sewer systems that also handle rainwater, he pointed out. Wastewater-treatment plants also have "bad days" when they aren't working efficiently, he added.
Sedlak and graduate student Lorien J. Fono figured out that monitoring the high-volume beta-blocker drug propranolol, which is used to treat high blood pressure, could provide evidence that raw sewage had entered a waterway (Environ. Sci. Technol. 2005, 39, 9244). Propranolol is a 50:50 mixture of R and S enantiomers, but in wastewater-treatment plants microbes preferentially degrade the R enantiomer, Sedlak noted. In field tests, the researchers have shown that water containing untreated sewage has an even proportion of the enantiomers, but water that was treated has less of the R enantiomer.
The technique could be particularly useful for discovering leaking sewer lines or tracking untreated water, and it could double as a warning for potentially harmful waterborne pathogens, Sedlak said. With further work, monitoring propranolol or a combination of contaminants could provide a quantitative measure of how much raw sewage entered a waterway.

Several presentations at Pacifichem addressed long-chain perfluoroalkyl compounds and their degradation products, which currently are at the head of the list of compounds of emerging concern. These chemicals are used in a wide array of consumer products ranging from pizza-delivery boxes and nonstick cookware to car parts and stain-resistant carpet. The inertness of the C-F bond helps provide the stain-, oil-, and water-resistance that makes these perfluoroalkyl compounds useful. But when the perfluorocarbon chain is seven carbon atoms or longer, the compounds resist degradation, accumulate in the environment, and are potentially toxic.
In one talk, chemistry professor Scott A. Mabury of the University of Toronto explained how and why perfluorinated carboxylic acids have been detected in people and wildlife around the globe, even in the remote Arctic region.
"This is a great story because we have an opportunity to potentially head off an environmental problem before most people even appreciate we have one," Mabury told C&EN.
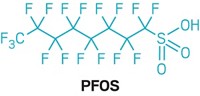
Perfluorooctane sulfonate (PFOS) was one of the key perfluorinated compounds originally observed in the environment in the late 1990s, Mabury explained. This compound was identified as a breakdown product of perfluorooctanesulfonamide, the key ingredient in 3M's Scotchgard brand of stain-protection products. 3M took a proactive step and voluntarily phased out the sulfonamide when faced with evidence that PFOS was accumulating in the environment and was potentially toxic to humans. EPA also issued regulations to limit U.S. manufacturing and imports of the sulfonamide.
Attention has now turned to perfluorocarboxylates, primarily perfluorooctanoic acid (PFOA), which also have been detected in people and animals around the world. EPA is evaluating whether PFOA should be classified as a likely human carcinogen.
Ammonium perfluorocarboxylates—mostly C8 and C9 compounds—are used in small amounts as surfactants to aid industrial production of polytetrafluoroethylene (DuPont's Teflon) and other fluoropolymers, Mabury added. Significant amounts of PFOA and other perfluorocarboxylates have been released during their production and during subsequent manufacturing processes that use them, but these emissions can't fully account for the spread of PFOA in the global environment, he said.
PFOA and most long-chain perfluoroalkyl compounds have low volatility, and their main route of transport is in water, he noted. That means that most PFOA emissions will end up in the oceans, where PFOA is detected. Some of the compounds are slowly transported around the world by ocean currents or perhaps more quickly by migrating fish and birds, as shown in studies of PCBs and mercury. Mabury began to suspect several years ago that the uniform distribution of perfluorocarboxylic acids observed across the remote Arctic region meant that there also must be an atmospheric piece to the puzzle.
He believes the real culprits are fluorotelomer alcohols (FTOHs), such as CF3(CF2)7CH2CH2OH. FTOHs are known from lab studies to degrade to the carboxylic acids. The alcohols are produced in much larger amounts than the perfluorocarboxylates and are used as building blocks to make perfluorocarbon surface-treated products. The alcohols are added to the backbone of various polymers and surfactants, which in turn are used to produce carpeting, clothing, food packaging, and paints and floor coatings.
Some FTOHs escape during manufacturing processes, Mabury said. "A fundamental question is whether FTOH emissions also arise indirectly from outgassing of residual material in products or from degradation of the perfluorinated products," he added.
In smog-chamber studies carried out with atmospheric chemist Timothy J. Wallington of Ford Motor Co., Mabury and his colleagues determined the atmospheric lifetimes of FTOHs and that they are oxidized to aldehyde intermediates by chlorine and hydroxyl radicals under UV light. The intermediate aldehydes undergo additional radical reactions to form peroxyl radicals, eventually leading to perfluorocarboxylic acids and other compounds. Lifetimes of FTOHs and the aldehyde intermediates are as long as three weeks, which is long enough for them to be transported in the atmosphere to the Arctic, Mabury said.
Metabolism studies in microbes and in rats, carried out by Mabury, graduate student Joyce A. Dinglasan, and postdoc Jonathan W. Martin, show that small amounts of FTOHs are transformed into PFOA and other perfluorinated carboxylic acids in living systems. These findings are important when potential human exposures are being considered. "Some of the intermediates may be more important toxicologically than the perfluorocarboxylic acids," Mabury noted.
Mabury, Derek C. G. Muir of Environment Canada, and their coworkers also have carried out a number of environmental-monitoring studies. These show that PFOA and other perfluorocarboxylic acids (chains up to C15) are in samples from the Great Lakes and in remote Arctic regions, including in polar bears, which are at the top of the food chain.
One of the group's latest projects is a modeling study led by Wallington that shows the smog-chamber results correlate to real atmospheric processes (Environ. Sci. Technol. 2006, 40, 924). The modeling also provides a quantitative estimate of how much FTOH-derived PFOA might be expected to be found in the environment.
On the basis of known production and emission rates of perfluoroalkyl compounds, Mabury said that about half a ton of perfluorocarboxylic acids per year might be deposited across the Arctic. That's a relatively small amount, but because PFOA can persist for years in the environment, the compound is expected to build up over time. Preliminary measurements by Mabury's group at locations in Canada place PFOA concentrations at about 1 ng/L (1 ppt), "which comes out pretty close to the model predictions," Mabury commented.
Mabury's group is also just finishing up work that shows unbound C6 to C14 FTOHs are present in up to about 4% by mass in some off-the-shelf carpet- and fabric-protection products, windshield-washing fluid, and other products. It appears the FTOHs are residual unreacted reagents, rather than compounds formed by cleaving off branches from the polymer or surfactant backbone, he said.

A recent review of the scientific evidence on perfluorocarboxylates in the environment corroborates many of the observations made by Mabury and his coworkers but draws a different set of conclusions. The study, carried out by Ian T. Cousins of Stockholm University, Robert C. Buck of DuPont, and their colleagues as part of a European Union program, is the first detailed accounting of the production history, direct and indirect emissions, and fate of PFOA and its homologs (Environ. Sci. Technol. 2006, 40, 32).
Advertisement
Most of the PFOA in the environment, including in the Arctic, likely is the result of PFOA releases dating as far back as the late 1940s, Cousins told C&EN. His conclusion, based on the study, is that eliminating FTOHs likely would have little future impact on PFOA and other acids in the Arctic. Cousins and his coauthors recommended "aggressive action" to eliminate direct sources of PFOA, such as plant emissions.
For DuPont's part, the company already has been working to cut PFOA emissions in fluoropolymer production in the U.S., with plans to reach a 98% reduction by next year. DuPont also recently reached a settlement with EPA over allegations that the company withheld health and safety information related to PFOA (C&EN, Dec. 19, 2005, page 10). As part of the settlement, the company will spend $5 million over three years for contract labs to evaluate the potential for nine unnamed DuPont products to break down into PFOA.
DuPont also has announced that it will remove more than 90% of FTOH residuals from its products by the end of 2007.
"If our overall theory on indirect sources is correct, with the elimination of residuals we should see a very rapid response in air concentrations and in the generation of perfluorocarboxylic acids in remote environments," Mabury said in an interview with C&EN. He allows that if the problem is the result of degradation of the polymers and surfactants rather than residual FTOHs, that makes for "a more intractable problem" that will need more work to resolve.
Either way, chemists are already at work finding environmentally friendlier next-generation replacements. 3M unveiled a new version of Scotchgard in 2003 that uses the short-chain compound perfluorobutane sulfonate, which doesn't accumulate in the environment and has been evaluated by EPA and is considered safe. Other companies and academic researchers also are coming out with replacement products that have been patented and are starting to enter the marketplace.
Mabury isn't opposed to the continued use of the perfluoroalkyl chemicals because of their high utility. In fact, he views his detective work as being complementary to the work by DuPont, EPA, and other scientists. "We would like to eventually give regulators and industry all the information they need to allow the chemistry to be optimized so that these fascinating and highly important chemicals can continue to be used," he told C&EN.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter