Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Vancomycin Redesigned
Antibiotic of last resort is modified to combat bacterial resistance
by Stu Borman
February 13, 2006
| A version of this story appeared in
Volume 84, Issue 7

The natural product vancomycin is an antibiotic of last resort, used when other antibacterial agents aren't effective. But some bacteria have developed resistance to vancomycin. Researchers have now designed and synthesized a revamped form of vancomycin that makes such resistance futile, or at least a lot more difficult.
Bacteria such as Staphylococcus aureus, a cause of food poisoning and a source of hospital infections, have developed resistance to a number of antibiotics but not to vancomycin???yet. When they do develop resistance to vancomycin, "as many suspect is just a matter of time, we will be in trouble," says chemistry professor Dale L. Boger of Scripps Research Institute.
In an effort to prepare for such an eventuality, graduate student Brendan M. Crowley and Boger reengineered vancomycin to make it much more resistance-proof and devised a way to synthesize the modified version from simple starting materials (J. Am. Chem. Soc., published online Feb. 4, dx.doi.org/10.1021/ja0572912).
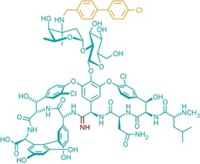
Vancomycin works as an antibiotic by binding to a peptidoglycan that is essential to the biosynthesis of bacterial cell walls. The most common form of resistance is due to a modification that changes a peptidoglycan amino acid from d-alanine to d-lactate. This mutation greatly reduces the affinity of vancomycin for the peptidoglycan, rendering it ineffective.
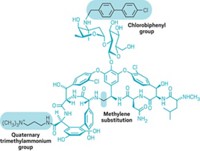
Crowley and Boger compensated with a comparably simple change to vancomycin. They synthesized an analog in which a carbonyl group positioned deep inside the vancomycin molecule has been converted to a methylene. This replacement enhances the compound's binding affinity for the modified peptidoglycan in vancomycin-resistant bacteria yet preserves a substantial amount of the antibiotic's affinity for the normal peptidoglycan in vancomycin-sensitive bacteria. The analog is thus 100-fold more active than vancomycin against vancomycin-resistant bacteria but only 3% as active as the drug against vancomycin-sensitive bacteria.
"This is an important breakthrough and will help medicinal chemists rationally design new antibacterial agents," says assistant professor of chemistry and biochemistry Yong Gao of Southern Illinois University, Carbondale, whose research interests include peptidoglycan-targeted antibacterial agents. "The model vancomycin analog in this paper is only available through chemical synthesis, which suggests that organic chemists can play unique and important roles in studying complicated biological systems."
In earlier work, Christopher T. Walsh, professor of biological chemistry and molecular pharmacology at Harvard Medical School, and coworkers had determined the molecular basis of vancomycin resistance to be a three order of magnitude drop in the drug's affinity for the modified peptidoglycan. Boger and coworkers studied the detailed interactions that caused this loss of affinity, tracing it to a missing hydrogen bond and an increase in lone-pair electronic repulsion.
They used "real mechanistic insight to probe an important therapeutic question in studies achievable only by remarkable practical mastery of the precepts of total synthesis of complex molecules," Walsh comments. Boger's group "may be the only one on the planet that could achieve this synthesis," he says. The study "establishes the principle for the first time that tinkering with the natural-product scaffold can give a molecule equally active against wild-type and resistant forms of this opportunistic bacterial pathogen."
In another sense, "it is a starting point for further work," Walsh notes. "An additional 10-fold gain in affinity would generate a usefully potent antibiotic to replace vancomycin." At present, "the total synthesis is not ready for process development nor for biosynthetic development," he says.
Boger says that his group is currently trying to improve analog activity and that the synthetic problem is solvable. One could possibly prepare the analog from vancomycin itself—"We just have not yet discovered a way to do it," he says. "Access to the drug by synthesis is not out of the question. A good process group might be able to work wonders with the basic route we developed. And a genetically engineered organism might be coaxed to produce analogs or analog precursors." Such efforts might lead to an even better antibiotic of last resort.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter