Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Bacteria Form Uranium Dioxide 'Pearls'
Cytochromes in bacterial slime convert soluble uranium to UO2 nanoparticles
by Stephen K. Ritter
August 16, 2006

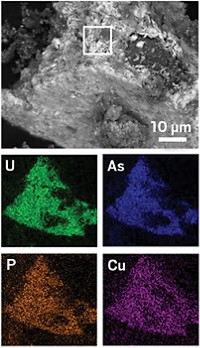
One prospect for bioremediation of groundwater contaminated with heavy metals is capitalizing on the ability of microbes to convert soluble metal ions into insoluble minerals that are immobilized in soil. A research team led by Matthew J. Marshall and James K. Fredrickson of Pacific Northwest National Laboratory has added to understanding of this process by partially unraveling the mechanism by which Shewanella oneidensis bacteria convert U6+ ions into 5-nm UO2 nanoparticles (PLoS Biol. 2006, 4, e268).
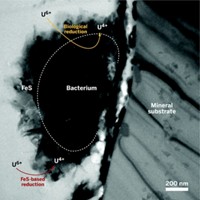
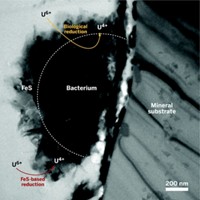
S. oneidensis was known to reduce uranium, iron, and other metals under anaerobic conditions by electron transfer via certain cytochrome enzymes. The bacterium plays an important role in the weathering of rocks and global cycling of soil nutrients.
The researchers developed mutant strains of the bacterium that enabled them to observe that UO2 nanoparticles are formed both inside and outside of the cells. They were able to pinpoint a specific cytochrome in the outer membrane of the cells that is responsible for electron transfer outside the cells. The cells release the cytochrome into an extracellular polymeric substance, a kind of slime produced by the bacterium, where the reduction primarily takes place.
The sticky slime serves not only as a medium for the reduction but also as a glue to bind the extracellular nanoparticles and possibly prevent them from reoxidizing back to U6+, Fredrickson says. This property could be the basis of a remediation process to halt underground migration of uranium and other heavy metals at contaminated industrial and nuclear waste sites, he adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter