Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Beyond The Periodic Table
Metal clusters mimic chemical properties of atoms
by Ivan Amato
November 21, 2006

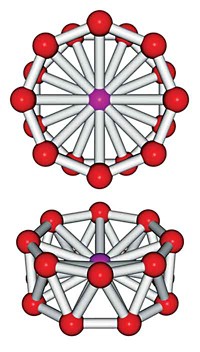
If Shiv N. Khanna is right, he and his colleagues have found a portal to another periodic table of sorts, this one populated by metal cluster "superatoms" that mimic the atoms of the original table. Their latest superatom, an Al7- cluster, behaves like a single multivalent germanium atom (Proc. Nat. Acad. Sci. USA, DOI: 10.1073/pnas.0608781103).

The scientists say that if someone can find ways of making large supplies of such superatoms, instead of the minuscule amounts in their gas-phase experiments, it might become possible to develop a superatom-based chemistry for creating new categories of catalysts, semiconductors, and other materials.
In the past few years, Khanna, a physicist at Virginia Commonwealth University, and Pennsylvania State University's A. Welford Castleman Jr., have made aluminum clusters that consist of exactly 13 or 14 atoms and have electronic structures and chemical traits like those of halogen or alkaline earth atoms, respectively. They also found that Al13- clusters exhibit a chemical aloofness akin to that of argon and other inert gases (C&EN, April 5, 2004, page 56).
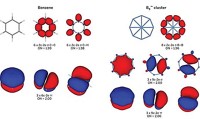
Such cluster-atom correspondences have fanned the notion that it might be possible to generate clusters that mimic all members of the periodic table. Now, with Al7- clusters in hand, that possibility seems more likely than ever, Khanna says.
"What is truly remarkable is that unlike previous members, this superatom exhibits multiple valence states, enabling it to form stable compound clusters when combined with diverse elements," the researchers write in their PNAS paper. The compounds they make from the Al7- cluster include Al7C- and Al7O-.
"For us, superatoms are those clusters that are fairly stable but mimic the chemical features of atoms in the periodic table," Khanna explains. "This extends the periodic table into a third dimension."
"These are tantalizing building blocks, but as yet, to my knowledge, no one has made macroscopic amounts," comments Mark Knickelbein, a cluster chemist at Argonne National Laboratory.
To make sense of their findings and to make predictions of clusters that would behave like new superatoms, the researchers summon the so-called Jellium model. This well-established model depicts the inside of a cluster as a homogeneous matrix of atomic nuclei and inner-shell electrons. This depiction results in a core of net positive charge that generates an electric potential by which valence, or bonding, electrons from the contributing atoms become organized.
Using this theoretical treatment, the researchers describe the electronic structure of clusters with the same nomenclature chemists have long used to describe the electronic structures of the elements. For example, the electronic structure of Al7-, which has 22 valence electrons, is written as 1s21p61d102s21f2. Khanna says his group is now pushing to discover more superatoms using magnesium, gold, boron, and other elements.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter