Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Auxin In Action
Crystal structure shows how plant hormone interacts with proteins
by Sarah Everts
April 9, 2007
| A version of this story appeared in
Volume 85, Issue 15
Fruit ripening, root branching, and even the flowering of some species are all nurtured by auxin, the plant world's most legendary hormone. Now researchers have finally figured out how this indole activates such a wide variety of plant processes (Nature 2007, 446, 640).
"It's amazing that plants all over the world are using auxin at this very moment," says research leader Ning Zheng, a biochemist at the University of Washington, Seattle. "And now we know the mechanism."
Charles Darwin was the first to be captivated by the action of this hormone, when he studied how plants reorient their leaves to grow toward light???yet another plant behavior that can be chalked up to auxin. Although auxin's molecular structure was identified in the 1930s, it took until 2005 before its biological receptor was finally pinpointed (C&EN, May 30, 2005, page 11). That receptor is a protein called TIR1, which shuttles proteins to degradation centers in the cell.
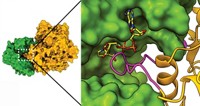
But this discovery left researchers scratching their heads about how TIR1's association with auxin could lead to a myriad of plant processes. The new X-ray crystal structure, solved by Zheng's graduate student Xu Tan in collaboration with Mark Estelle's group at Indiana University, provides a surprisingly simple answer.
At high enough concentrations, auxin nestles into a groove on TIR1. The presence of auxin dramatically enhances the association of TIR1 with a short peptide found in many proteins that block gene transcription.
The auxin-TIR1 duo pulls these gene transcription repressors away from their normal business and allows blocked genes to be turned on. An inositol hexakisphosphate cofactor located near auxin in the TIR1 binding pocket also lends a sticky hand.
Auxin can turn on such a variety of genes because the peptide that the TIR1-auxin combo binds is present in many different transcription repressors. By targeting the degradation of diverse repressors, auxin activates diverse plant processes.
It's a "beautiful" study, comments Bonnie Bartel, a geneticist at Rice University. "Not only is it the first plant hormone receptor-ligand structure solved, it is an entirely new type of receptor for protein degradation."
Typically, proteins are targeted for degradation by undergoing a cascade of phosphorylations. These chemical signals prompt the covalent attachment of ubiquitin proteins that then destine the protein to the cell's landfill site. The use of a noncovalent ligand such as auxin as the destruction signal is novel, notes Judy Callis, a biochemist at the University of California, Davis.
In fact, this new mechanism to target proteins for degradation may be operative in all eukaryotic cells, from plants to humans, Callis says. "Researchers in other systems should be aware of this possibility."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter