Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Acid catalysis in basic solution
April 9, 2007
| A version of this story appeared in
Volume 85, Issue 15
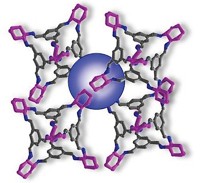
By confining a reactant inside electrostatically tuned molecular cages, chemists have performed acid catalysis in basic solution (Science 2007, 316, 85). Michael D. Pluth, Robert G. Bergman, and Kenneth N. Raymond of the University of California, Berkeley, show that a water-soluble, tetrahedral metal-ligand assembly (shown, metal ions are green) thermodynamically drives the protonation of a guest molecule (space-filling model) trapped inside its highly charged cavity. The researchers exploit this host-induced shift in the guest's ability to accept a proton to do acid catalysis in basic solution. In the presence of catalytic amounts of the host, orthoformates [HC(OR)3, R = alkyl] in basic solution are trapped inside and protonated by water, resulting in rapid hydrolysis of the otherwise stable-to-base orthoformate. A similar strategy could be used to hydrolyze other acid-sensitive molecules in basic environments, the researchers note. The host's ability to select appropriately sized substrates is of particular interest, they add, because size selectivity is "often used by nature but rarely incorporated into standard homogeneous or heterogeneous catalysis."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter