Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Sharper image of fatty acid synthase
April 16, 2007
| A version of this story appeared in
Volume 85, Issue 16
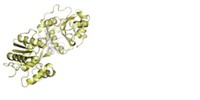
The gargantuan 2.6-megadalton protein complex that catalyzes the iterative assembly of fungal fatty acids has just come into sharper focus. Nenad Ban and colleagues at the Swiss Federal Institute of Technology, Zurich, slimmed down their previous 5.0-?? resolution X-ray structure of the synthase to 3.1-?? resolution and suggest how this monster enzyme catalyzes a total of 42 reaction steps to create an 18-carbon chain (Science 2007, 316, 254). The protein has two reaction chambers with three full sets of active sites, so six fatty acids can be synthesized simultaneously. A special carrier protein shuttles intermediates around the reaction chamber; the fatty acid chain length increases by two carbons with each cycle. A second paper by Ban's group reveals that the carrier protein places a fatty acid intermediate in a ketoacyl synthase active site as if the intermediate were at the end of an opening switchblade (Science 2007, 316, 288).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter