Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
One-Pot Route To Sugar Building Blocks
Method avoids time-consuming synthetic steps
by Stu Borman
April 23, 2007
| A version of this story appeared in
Volume 85, Issue 17

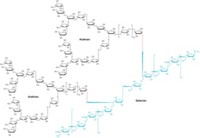
WHEN CARBOHYDRATE CHEMISTS want to join simple sugars to form oligosaccharides, each sugar building block has to be laboriously prepared in multiple steps. Now, a research team has achieved the long-sought goal of preparing the building blocks through one-pot reactions.
The achievement is the fruit of a seven-year effort by chemistry professor Shang-Cheng Hung, graduate student Cheng-Chung Wang, and coworkers at National Tsing Hua University and Academia Sinica, both in Taiwan (Nature 2007, 446, 896).
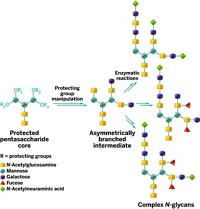
It's important to control sugar-joining, or glycosylation, reactions so they take place at only one of the reactive hydroxyl groups on each sugar unit. Protecting the other hydroxyls is difficult, requiring a series of time-consuming protection, reaction, and cleanup steps.
Hung and coworkers identified a catalyst and sets of compatible reagents that, in one pot, act on a fully protected monosaccharide so only one hydroxyl group at most is left unmasked and free to undergo glycosylation. The researchers used the one-pot reactions to synthesize five types of glucose building blocks: four with different free hydroxyls and one (for use at chain ends) with none. They used the technique to synthesize a small library of flu-virus-binding trisaccharides much more quickly than would otherwise have been possible.
Protecting-group patterns often need to be modified "before a high-yielding and stereoselective glycosylation is achieved," comments chemistry professor Geert-Jan Boons of the University of Georgia, Athens. The new technique "is novel and can provide a wide range of selectively protected monosaccharides in a short period of time, which will be very valuable for complex oligosaccharide synthesis."
Boons points out that applicability of the technique to a wide range of sugars has yet to be demonstrated. But Wang notes that he and his colleagues have obtained good results with glucose, mannose, galactose, and azidodeoxyglucose and are currently working on additional monosaccharides.
Chemist Ole Hindsgaul of Carlsberg Laboratory, in Copenhagen, says "it takes only about one day's work" to prepare a purified building block with the new technique, whereas conventional approaches typically would take a week or two. The new approach should "reduce from months to weeks the preparation of most sought-after two- to four-unit oligosaccharides," he adds.
Carbohydrate chemist Peter Seeberger of the Swiss Federal Institute of Technology, Zurich, comments that obtaining "differentially protected monosaccharide building blocks is currently the bottleneck for chemical synthesis" and that the new study "addresses this need in an innovative manner."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter