Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Rhenium diboride stands up to diamond
April 23, 2007
| A version of this story appeared in
Volume 85, Issue 17
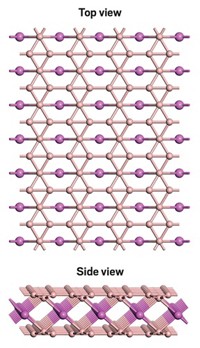
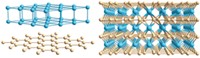
With its milquetoast metallic appearance, rhenium diboride (ReB2) probably won't usurp the place of diamond as a girl's best friend. But the superhard material could replace diamond in certain industrial applications, thanks to its ultra-incompressible nature and its relatively simple synthesis. Sarah H. Tolbert, Richard B. Kaner, and coworkers at UCLA prepared the metallic form of ReB2 in bulk quantities under ambient pressure via an arc-melting procedure (Science 2007, 316, 436). The material's average measured hardness is comparable to that of other superhard materials, such as cubic BN or B6O, which must be made at extreme pressures and temperatures. It falls short of being as hard as diamond, but Tolbert and Kaner believe their measurement represents only a lower limit of ReB2's hardness. Rhenium diboride can scratch the surface of natural diamond, however, indicating that it's one of the hardest materials known.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter