Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Stripped-Down Synthesis
Phil Baran is spearheading natural products synthesis without protecting groups
by Bethany Halford
June 4, 2007
| A version of this story appeared in
Volume 85, Issue 23

WHAT DO Botticelli's Venus and Phil Baran's molecules have in common? Both are born of the sea—Baran works on the synthesis of marine natural products—and both are, well, basically naked.
Baran, an associate professor at Scripps Research Institute in La Jolla, Calif., is taking a stripped-down approach to making these complex marine molecules by minimizing the use of protecting groups-chemical shields that preserve sensitive functional groups during certain transformations. In doing so, he and his coworkers have demonstrated how to unlock a molecule's innate reactivity, leading to some elegant total syntheses.
"It is some of the most impressive, efficient, and interesting synthetic chemistry that's being published right now in terms of structurally complex natural products," comments John A. Porco Jr., a chemistry professor at Boston University.
Using protecting groups seems inevitable when synthesizing complex molecules with multiple functional groups. It's easier to tinker with one part of an elaborate molecule when protecting groups shield the rest of it.
Baran's Eight Guidelines
Here are some suggestions for achieving more efficient syntheses.
- Avoid redox reactions that don't form C-C bonds.
- Maximize the number of C-C bond-forming reactions at each step.
- Choose disconnections that create the most reasonable convergent synthesis.
- Increase the overall oxidation level of each intermediate linearly during the assembly of the molecular framework, except when an asymmetric reduction would be strategically beneficial to the overall synthesis.
- Use cascade or tandem reactions wherever possible to maximize efficiency.
- Exploit the innate reactivity of functional groups so that the use of protecting groups may be minimized or eliminated altogether.
- Try to invent methodology that facilitates the previously mentioned guidelines and uncovers new aspects of chemical reactivity.
- Keeping the aforementioned in mind, incorporate biomimetic pathways whenever possible for target molecules that are natural products or derived from natural products.
But using protecting groups comes at a cost. A protecting group adds two steps to an overall synthetic scheme—one to put the group on and one to take it off. That can translate into reduced yields and increased use of solvents. Also, chemists at times have been unable to remove a protecting group near the end of a long reaction sequence. This can be a synthetic chemist's worst nightmare. "Years of work are frequently ruined in this way," Porco notes.
Protecting groups haven't always been de rigueur in organic synthesis. In the early days, chemists had to craft syntheses using molecules with all functional groups exposed. Since the advent of protecting group chemistry, chemists' arsenal of reagents has grown and so has the size and complexity of the natural products they synthesize.
"The concept of synthesis without protecting groups is as new and revolutionary as the idea of free love," remarks Dirk Trauner, a chemistry professor at the University of California, Berkeley. "It may work well for some, but it is hardly universally applicable." Trauner adds, however, that "Baran's syntheses certainly set a new standard of boldness and elegance."
Baran has been thinking about the overuse of protecting groups since his undergraduate days. He remembers how one of his classmates was once singled out for creative use of a protecting group. The incident, he recalls, planted a seed of skepticism in his mind. He thought, "Should we really be praising students for using protecting groups?" Rather, he remembers thinking that the use of a protecting group was something to be avoided.
There can be an added benefit to not using these chemical shields. "The chemistry that is enabled by having the molecule in its raw or undressed state can expose the innate reactivity of the molecule," Porco explains. "That, I think, can really lead chemists into new reactivities that we might miss if we had dressed the molecule with protecting groups."
"It's not a gimmick or some sort of creative salesmanship," Baran adds. "The deliberate exclusion of protecting groups is a way to do synthesis. Is it going to work every time? No, of course not." But the point, he says, is to recognize the reactivity native to the system instead of its individual components.
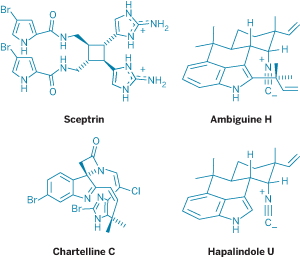
In Baran's recent synthesis of sceptrin and several other members in its family of dimeric pyrrole-imidazole alkaloids, his group managed to make molecules containing 10 nitrogen atoms on a gram scale using just one protecting group at the outset of the synthesis (J. Am. Chem. Soc. 2007, 129, 4762).
Instead of masking the molecule's innate chemical reactivity, Baran notes, the approach allowed them to explore the inherent chemistry of the molecule's 2-aminoimidazole heterocycles. In the process, the group discovered a new method for α-chlorinating ketals, a new method for asymmetric cyclobutane synthesis, and useful interconversions of 2-aminoimidazoles.
In another example, Baran and graduate student Thomas J. Maimone use their stripped-down approach to make (+)-ambiguine H in 10 steps without relying on any protecting groups (Nature 2007, 446, 404). The key to the synthesis was to use the innate reactivity of the molecule's indole moiety. Bucking the conventional wisdom, they left the indole's N-H bond unprotected and used reactions that relied on the free functional group.
The most dramatic example is the two-step conversion of (–)-hapalindole U to (+)-ambiguine H. Here, by leaving the indole and fragile isonitrile unprotected, they were able to exploit the natural reactivities of both functional groups and accomplish five transformations in one pot. "We were pretty excited that all those things happened in that same reaction flask," Maimone says.
Of course, not every reaction in the Baran lab follows such a meticulous design. "We try to take as much guidance from happenstance as we can," says graduate student Noah Z. Burns. "When things go wrong, we try not to see it as a failure. A lot of information can be gained from those empirical failures. We try to harness them."
Minimizing the use of protecting groups isn't the only way Baran's group is trying to streamline synthesis. The group tries to follow eight general guidelines when designing their syntheses. For example, the overall oxidation level of intermediates should escalate linearly as the molecule is assembled. And disconnections—sites where the researchers break the target molecule to figure out which building blocks and reactions are needed to put the molecule together—should be biomimetic when it's strategically advantageous.
"All these ideas that we put forward aren't unique to us," notes graduate student Jeremy M. Richter. "But we show that applying them can make synthesis more efficient."
"We're always striving for higher efficiency and, specifically, shorter reaction sequences," Burns adds. Any group "can make a molecule, given enough time and manpower on a project."
"One of the things I found helpful with Phil's philosophy is to look at the easiest disconnections," says graduate student Ryan A. Shenvi, who completed a synthesis of chartelline C (J. Am. Chem. Soc. 2006, 128, 14028).
"Don't worry about whether the chemistry is known. Don't worry about whether you can fit this into your synthetic arsenal," Shenvi advises.
"From a strategic standpoint, what are the easiest bond disconnections to make? As a result of that, we stumbled across some chemistry we would never have predicted." In particular, Shenvi points to the final decarboxylation step on an sp2-hybridized center in the chartelline C synthesis.
Synthetic efficiency has long been one of the primary aims of green chemistry. Baran's synthetic approach without protection and deprotection steps is "ingenious" and elegantly illustrates the principles of green chemistry, says Chao-Jun Li, a green chemistry expert at McGill University, in Montreal.
Kim Albizati, a classically trained synthetic organic chemist and the chief executive officer of the San Diego-based green chemistry firm BioVerdant, agrees that Baran's synthetic strategy is very green. "In judging the greenness of the approach, you have to consider three levels: operational, tactical, and strategic," he explains. Operationally and tactically, the routes aren't green at all, as they still employ chromatography and lots of solvents. But, Albizati continues, Baran's system-specific syntheses—schemes designed around a specific molecule rather than a specific reaction—really shine. That approach, he says, is "nearly always a strategically superior way to put molecules together."
"System-specific chemistry has its own kind of beauty," Albizati says. "Designing and executing a truly practical way to make a complex molecule in scientifically significant amounts is underrated and unbelievably important to science," he notes. "Put another way, being first in synthesis is not as important as being best."
Baran, for his part, is more understated about his work. He hopes that the deliberate elimination of protecting groups will advance organic synthesis, perhaps providing a new way to think about approaching dauntingly complex molecules.
Reporter's Notebook
Life In The Baran Lab
One might imagine that, working in sunny La Jolla, Calif., the students in Phil Baran's lab contemplate their syntheses while looking out upon sea lions frolicking in the great Pacific expanse. But aside from the marine natural products swirling away in reaction flasks, the only thing that evokes the nearby ocean is the pirate's flag in Baran's lab that bears the slogan "Commitment to Excellence."
The Baran lab, on the ground floor of Scripps Research Institute, does have a view, thanks to La Jolla's hilliness. One window looks out on a loading dock, and the other frames a retaining wall. The retaining wall side of the lab gets about four-and-a-half minutes of direct sunlight each day, according to graduate student Ryan A. Shenvi. "That's our daily vitamin D ration," he jokes. On the plus side, he adds, they're close to the NMR spectrometer and coffee cart.
At 29, Baran isn't much older than his two dozen or so graduate students and postdocs. When most of them were starting college, he was starting graduate school at the very institution where he is now an associate professor. Baran has a reputation as a wunderkind, and he's not particularly comfortable with that. Rather than expound on his achievements, Baran would prefer to let his graduate students do the talking.
"It's the students that keep you happy," Baran says. "Their eagerness to learn and fearlessness gets you up and out of bed every morning."
"When I interviewed at Scripps, I didn't even know about Phil because he was finishing up his postdoc at Harvard," Shenvi says. But he was intrigued by Baran's approach to total synthesis-one that eschews very long synthetic protocols that are impractical and low-yielding. "For an incoming student, these long, monumental syntheses were extremely daunting, especially the idea of pushing material through 30 steps to provide late-stage intermediates for exploratory work."
That's not to say they don't work hard in the Baran lab. It's not uncommon for students to work 14 hours per day. Baran also holds "boot camp" group meetings where students dissect complex molecules on paper and then discuss the retrosyntheses as a group.
"One of the things I think Phil is really good at is having a sense of what is new, what is novel, and what needs to be discovered in our field," remarks graduate student Noah Z. Burns. "It's not an easy thing to do in a mature field."
- Stripped-Down Synthesis
- Phil Baran is spearheading natural products synthesis without protecting groups
- Reporter's Notebook
- Life In The Baran Lab
- Baran's Eight Guidelines
- suggestions for achieving more efficient syntheses



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter