Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Masks Unveil New Synthetic Routes
Protection schemes enable iterative use of organic coupling reaction
by Stu Borman
June 18, 2007
| A version of this story appeared in
Volume 85, Issue 25
MASKS ARE OFTEN USED for nefarious purposes, like robbing banks. But the Lone Ranger—the fictional American lawman who wore one while fighting injustice with his faithful partner, Tonto—used his mask for a good purpose.
A couple of scientific teams, working independently, have recently been using masks as a force for good as well—good chemistry, in their case. They've used masks to devise new synthetic routes to a variety of potentially useful arene oligomers and organic small molecules.
The new approaches, in which protected haloboronic building blocks are combined iteratively, could be important for the organic synthesis of compounds with applications in materials science, pharmaceutical chemistry, and other areas, says chemistry professor Michinori Suginome of Kyoto University, in Japan, leader of one of the groups.
The other researcher, assistant professor of chemistry Martin D. Burke of the University of Illinois, Urbana-Champaign, notes that his group's new strategy "has significant potential to simplify the process of complex-molecule synthesis. It's remarkably general, and we expect it will have broad impact in the pharmaceutical and academic synthesis communities."
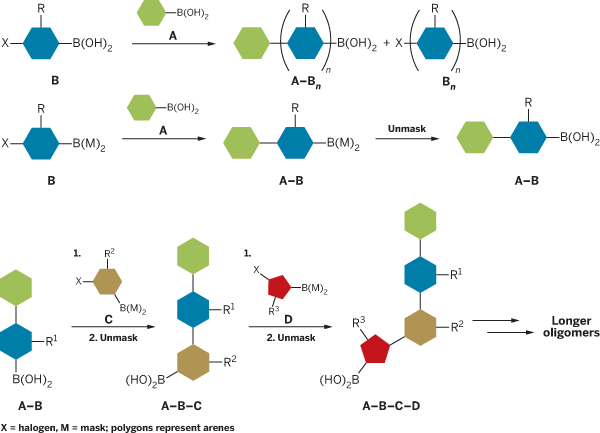
The two groups' techniques are based on a common concept, but differ in implementation. Both use a process called Suzuki-Miyaura coupling to synthesize organic compounds. In Suzuki-Miyaura coupling, an organoboronate (A) and an organohalide (B) combine to yield an A-B product, in which the boronate and halogen have been replaced by a carbon-carbon bond.
Suzuki-Miyaura coupling can conceivably be used iteratively to produce larger, more complex products (such as A-B-C-D) if an organoboronate (A) is reacted sequentially with a series of bifunctional organohaloboronates (B, C, and D). In each reaction step, the organohaloboronate would react at its halogen, leaving its boronate free to react with the halogen of the next organohaloboronate.
But couplings with such bifunctional organohaloboronates yield undesired by-products (such as A-B-B-B) because the bifunctional reagent reacts with itself. Formation of these by-products has prevented iterative use of the coupling reaction.
Suginome and coworkers Hiroyoshi Noguchi and Kosho Hojo addressed this problem by designing a mask for the boronyl group on the bifunctional organohaloboronate (J. Am. Chem. Soc. 2007, 129, 758). The masking group, 1,8-diaminonaphthalene, renders the boronyl group inactive, enabling the organoboronate to react with the bifunctional reagent's halogen to form A-B addition products without any oligomerization. After the coupling reaction, the mask is removed with aqueous acid, and the process can then be repeated to yield A-B-C-D-type products.
"As far as we are aware, a masking-unmasking strategy has never been used to control reactivity of organoboronic acids in the coupling reaction," Suginome and coworkers write. They demonstrate the process by using it to synthesize a series of biaryl compounds and to create higher arene oligomers in an iterative manner.
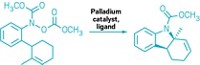
In an independent study, Burke and graduate student Eric P. Gillis aimed to synthesize complex small molecules, which are more delicate than arene oligomers. This necessitated developing a boronate mask that could be removed with mild reagents. The masking technique they developed accomplishes this by using a trivalent ligand to attenuate the reactivity of the boronates on organohaloboronates (J. Am. Chem. Soc. 2007, 129, 6716).

"To the best of our knowledge, this type of reactivity attenuation is unprecedented" in this type of system, Burke says. "Our ligand can be removed using extremely mild conditions—for example, even aqueous sodium bicarbonate—which is critical for applications to small-molecule synthesis."
Burke and Gillis demonstrated the approach by using it to synthesize the natural product ratanhine. Burke says he believes this to be the first example of a small-molecule natural product synthesis in which all the building blocks are assembled using only a single reaction in an iterative manner.
Chemistry professor James M. Tour of Rice University, Houston, who specializes in carbon-carbon bond-forming reactions, comments that both groups have devised "very useful strategies for the synthetic organic chemist to use with an important class of coupling reactions."
Polymer chemist A. Dieter Schlüter of the Swiss Federal Institute of Technology, Zurich, who has used Suzuki-Miyaura coupling extensively, says the two groups' discoveries "mark a new level of applicability of one of the most important methods of carbon-carbon bond formation."
Suginome says he hopes that "in the near future these masking groups are widely and complementarily used in organic synthesis by those who wish to obtain target molecules quickly and reliably." It's clear that his group and Burke's are using masks, in a Lone Ranger-like manner, to fight synthetic injustice, or at least synthetic limitations.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter