Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Sweet Routes To Sustainability
Catalytic reactions convert sugars from biomass into renewable fuel and feedstock
by Rachel Petkewich
June 25, 2007
| A version of this story appeared in
Volume 85, Issue 26
Many scientists expect that the fuels of the future will be made from carbohydrates found in plant material (biomass), but the fuels of choice and how they will be produced remain big question marks. Offering another potential production option, two research groups—one at Pacific Northwest National Laboratory (PNNL) and one at the University of Wisconsin, Madison—have independently reported two catalytic methods to convert biomass-derived sugars into renewable fuel and feedstock (Science 2007, 316, 1597; Nature 2007, 447, 982).
"These two papers clearly advance the field of biomass conversion to liquid fuel by offering innovative solutions to steps of the overall process," says Harold H. Kung, a professor of chemical and biological engineering at Northwestern University. "Their work helps clarify the vision and bring the possibility of large-scale biofuel introduction one step closer to reality."
The new catalytic methods do not rely on fermentation, which is currently used to manufacture bioethanol.

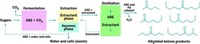
PNNL's Z. Conrad Zhang and colleagues convert glucose to 5-hydroxymethylfurfural. HMF itself is not a good candidate for a transportation fuel because its boiling point is too high. But HMF and its derivatives are multipurpose intermediates that could serve as fuel precursors or replace petroleum-based building blocks that are used to make some plastics, pharmaceuticals, and fine chemicals but are rarely used in industry due to high costs.
The PNNL researchers obtained a moderate yield (70%) of HMF, using a chromium chloride catalyst in 1-ethyl-3-methylimidazolium chloride, an ionic liquid solvent. By using fructose instead of glucose, they were able to produce HMF in higher yield (90%). The mechanism for glucose conversion remains unclear, but the researchers have preliminary ideas about what might be happening.
The Wisconsin group has taken its sugar-transformation process a step beyond HMF. James A. Dumesic and colleagues report selective conversion of fructose to HMF and then to 2,5-dimethylfuran (DMF). Fructose can be prepared from glucose or produced directly from biomass. DMF has potential for use as a transportation fuel and could be better than ethanol because it has a 40% higher energy density and is less volatile.

Dumesic and colleagues used a biphasic reactor for acid-catalyzed dehydration of fructose. HMF was extracted from the reactor with butanol. Over a copper-ruthenium catalyst, HMF underwent hydrogenolysis, where hydrogen is used to remove two oxygen atoms in the form of water, to produce a moderate yield of DMF.
Although several experts commend the new catalytic reactions, Joseph J. Bozell, a biomass chemist at the University of Tennessee, Knoxville, says, "there's still quite a bit of work to be done before these approaches show up in a commercial biorefinery."
In addition, Pierre Gallezot, a research director at France's National Center for Scientific Research (CNRS), points out some economic and environmental drawbacks. Processes that use ionic liquids can be expensive, and some acids and chromium catalysts are far from green chemistry, he says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter