Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Force affects chemical reactions
July 9, 2007
| A version of this story appeared in
Volume 85, Issue 28
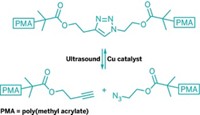
In a recent letter (C&EN, May 21, page 2), David K. Lewis and John E. Baldwin suggest an alternative explanation for the experimental observations reported in our article "Biasing Reaction Pathways with Mechanical Force" (Nature 2007, 446, 423).
Through a careful series of experiments and appropriate controls, we reported that when located within long polymer strands, the trans and cis isomers of a 1,2-disubstituted benzocyclobutene undergo ultrasound-induced electrocyclic ring opening in a formal conrotatory and formal disrotatory process, respectively. We specifically noted in Nature that the occurrence of the reaction depends strongly on the length of the polymer (see the data in Figure 3d of our publication). In particular, no significant reaction is observed when the mechanophore is incorporated into low-molecular-weight polymers.
Our data clearly showed that small-molecule analogs do not exhibit the observed reactivity. In the Nature publication we wrote, "A reaction promoted purely by thermal energy is not expected to have such a molecular weight dependence." This control experiment is crucial to our conclusion that force accelerates and alters the course of chemical reactions.
Lewis and Baldwin suggest that our ultrasound experiments were not "anything other than the expected result of thermal excitation to very high temperatures." While it is correct that extremes of pressure and temperature are locally produced in ultrasonic fields, we write to note that understanding the structural dependence of the data in our paper requires more than a suggestion of nonspecific thermal activation. It is difficult to believe that short polymer chains experience a different thermal bath than do long polymer chains under the conditions of our experiments.
We would be interested in any alternative explanation that does account for all of our data. However on the basis of all of the information in our publication, we stand behind the originally stated conclusions.
Jeffrey S. Moore
Nancy R. Sottos
Scott R. White
Urbana, Ill.
Charles R. Hickenboth
Pittsburgh


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter