Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Low-Cost Catalysis
Inexpensive MoS2 mimics precious-metal catalyst
by Mitch Jacoby
July 9, 2007
| A version of this story appeared in
Volume 85, Issue 28

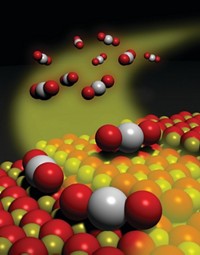
In work that could lead to economical substitutes for precious-metal catalysts, researchers in Denmark have produced hydrogen from water through a reaction catalyzed by a low-cost metal sulfide.
The unique surface properties of platinum, ruthenium, and other metals located in the same region of the periodic table endow those materials with the ability to catalyze numerous chemical reactions. They are widely used, for example, in automotive emissions cleanup and fuel-cell processes. Nonetheless, the metals' high cost has long motivated scientists to search for less expensive substitutes.
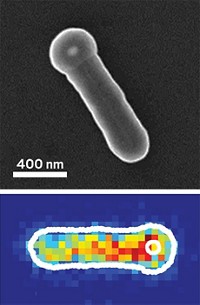
Using synthesis methods to control the size and morphology of single-layered, flat molybdenum disulfide nanoparticles, scientists at the Technical University of Denmark, in Lyngby, have demonstrated that the particles can catalyze the hydrogen evolution reaction (2H+ + 2e- → H2) in solution (Science 2007, 317, 100). They also have determined that this reaction occurs along the perimeter (edge) of the particles, a detail with both theoretical and practical value.
The gas-evolution reaction, which lies at the heart of solar-energy-driven hydrogen production via water splitting and runs in reverse in fuel cells, is a prime example of the type of reaction catalyzed by noble metals. Earlier theoretical work suggested that the edges of nanoparticulate MoS2 could catalyze the reaction, but until now that prediction had not been verified conclusively.
To prepare the nanoparticles, postdoc Thomas F. Jaramillo, physics professor Ib Chorkendorff, and their coworkers used vapor deposition methods and heat treatments to react molybdenum with hydrogen sulfide on a gold surface. Through judicious choice of synthesis conditions, the team was able to control the extent of particle sintering and thereby systematically vary the particle size and the relative numbers of atoms that reside within the terrace (the open crystal face) and along the nearly atomically thin edge. After analyzing the MoS2 samples using scanning tunneling microscopy, the team measured the particles' catalytic activity in electrochemical cells and determined that hydrogen evolution correlates linearly with the number of nanoparticle edge sites.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter