Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Methylmercury Detox
Hg complex provides clues to enzymatic breakdown of toxic compound
by Stephen K. Ritter
July 16, 2007
| A version of this story appeared in
Volume 85, Issue 29
Whether it's round, wrinkled, or rectangular, a polymeric particle's shape can strongly influence its properties. But there's been no simple way to control these shapes, until now.
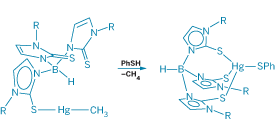
ACCUMULATION OF extremely toxic methylmercury in the environment—particularly in fish—has triggered an effort by scientists to unravel the process by which a set of bacterial enzymes capture and then detoxify the compound. In a new development, Jonathan G. Melnick and Gerard Parkin of Columbia University report a synthetic mercury complex that provides insight into how one of these enzymes catalyzes cleavage of the Hg-C bond (Science 2007, 317, 225). The finding is expected to boost efforts to genetically modify plants to sequester HgCH3+ for environmental cleanup.
In nature, microbes synthesize HgCH3+ from naturally occurring Hg2+, as well as from mercury released in the emissions of coal-fired power plants. Organomercury compounds are toxic because the metal has a high affinity for sulfur, in particular the sulfur of thiol (-SH) groups in cysteine units of proteins. Once the mercury binds, the normal function of the proteins is disrupted.
Bacteria resistant to HgCH3+ toxicity produce an enzyme named MerB, which has three cysteine residues in its active site that are known to be crucial for cleaving the Hg-C bond. But the exact way in which MerB coordinates to HgCH3+ and the "intimate details of the reaction mechanism" have been a mystery, Parkin says. (A second enzyme, MerA, reduces the resulting Hg2+ to less toxic elemental mercury.)
Melnick and Parkin thus set out to decipher the mechanism of action of MerB. They synthesized bulky alkylmercury complexes by coupling a methylmercury or ethylmercury group to a sulfur-rich imidazolylborate ligand. Like MerB, the ligand contains three key sulfur atoms for binding mercury.
From crystal structure data and nuclear magnetic resonance spectral studies of these complexes, the researchers determined that HgCH3+ or HgCH2CH3+ is bound to one sulfur donor in the solid state via linear S-Hg-C bonding. But in solution, the complexes exist in rapid equilibrium with analogs in which the mercury atom binds to two or three ligand sulfur atoms.
As a further test, they reacted their complexes with phenylthiol to simulate a cysteine thiol group in MerB. Introducing phenylthiol into the system results in cleavage of the alkyl group.
The researchers conclude that the ability of the mercury atom to coordinate to multiple sulfur atoms sets up cleavage of the Hg-C bond. They envision that, for MerB, one cysteine is required to coordinate HgCH3+ in a linear fashion, a second cysteine is required to activate the Hg-C bond toward cleavage, and a third cysteine is required to sever the bond.
Melnick and Parkin "provide an elegant atomic-level description for the facile cleavage of a carbon-mercury bond," notes James G. Omichinski of the University of Montreal in a Science commentary. Their observations provide valuable insight into the basic mechanism of MerB's activity, he adds. Considerable work remains to be done, but understanding this mechanism "is essential to efforts to reengineer MerB to improve its catalytic efficiency for the bioremediation of methylmercury," Omichinski writes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter