Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Tailor-Made Nanostructures
New methods offer precise control of polymeric micelles
by Bethany Halford
August 6, 2007
| A version of this story appeared in
Volume 85, Issue 32

CONTROLLING THE FORMATION of complex polymeric nanostructures is no easy task. As the polymer molecules self-assemble, they tend to form particles in an unruly mess of different shapes and sizes. Two new advances offer scientists the ability to tailor these properties, thereby offering nanostructure design strategies that could be useful for a number of applications, including drug delivery and nanolithography (Science 2007, 317, 644 and 647).
Both strategies use block copolymers, which contain segments, or blocks, of different compositions and solubilities. In solution, these amphiphilic molecules organize into micelles, where the insoluble segments come together to form a core surrounded by a corona of the more soluble segments. A group led by Ian Manners of the University of Bristol, in England, and Mitchell A. Winnik of the University of Toronto demonstrated that they can control the length of cylindrical micelles using a novel polymer growth mechanism.
The researchers first prepare short, cylindrical polyferrocenyldimethylsilane-containing block copolymers in a specific solvent. Then they add the same polymer to a different solvent in which both types of polymer segments are soluble. With this method, the researchers can dial in the length of the micelles: The more polymer they add, the longer the micelles become.
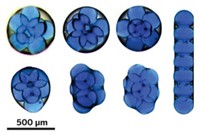
In the other work, a team led by Karen L. Wooley of Washington University, St. Louis, and Darrin J. Pochan of the University of Delaware used electrostatic interactions to manipulate how charged, amphiphilic block copolymers aggregate into supramicellar assemblies. The researchers employ a triblock copolymer that contains a carboxylic acid unit. By varying the solvent or the counterion to the acid groups, they can drive the organization of the block copolymers into specific nanostructures.
"By controlling the kinetics of assembly, we can get many nanostructures from the same molecule, as opposed to having to design completely new molecules to get different nanostructures," Pochan explains.
Both reports "demonstrate how the self-assembly of block copolymer amphiphiles can be controlled with unprecedented precision and how the marriage of organic, inorganic, and polymer chemistries can yield fascinating new and exotic nanometer-scale assemblies," notes the University of Minnesota's Marc A. Hillmyer in an accompanying Science commentary.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter