Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Biotechs Rely On Old Products
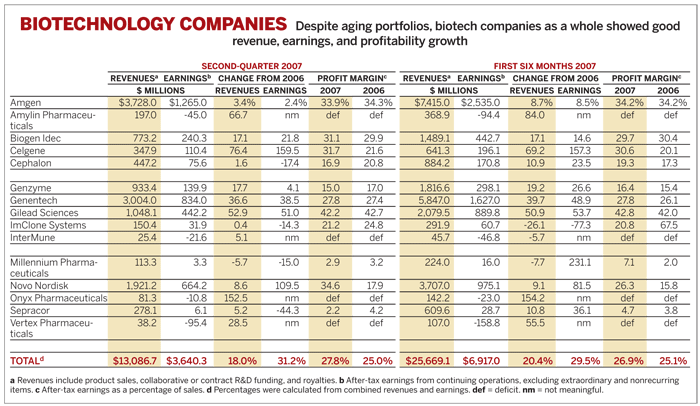
Second-quarter financial results reflect aging portfolios at big biotech companies
by Lisa M. Jarvis
August 20, 2007
| A version of this story appeared in
Volume 85, Issue 34
MAJOR BIOTECH COMPANIES reported relatively lackluster results in the second quarter, which was notable primarily for the absence of new revenue streams. With few new products, Amgen, Genentech, and Biogen Idec are leaning on a handful of older drugs to achieve the lofty growth investors have come to expect. Such a strategy, the companies are learning, can be swiftly undermined when a product comes under pressure.
On average, second-quarter earnings at the 15 companies tracked by C&EN increased by 31.2% over the same period in 2006, based on an 18.0% rise in revenues. Average profit margins increased to 27.8%, compared with 25.0% in the second quarter of 2006. For the first half of the year, earnings were up 29.5%, while revenues rose 20.4%. The averages seem superb, but they are due to rapid growth of small firms and belie the difficulties of major companies.
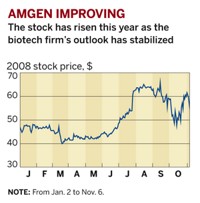
Amgen had the toughest quarter, with earnings growing just 2.4% to $1.3 billion, based on revenues of $3.7 billion, up 3.4%.
"This has been a difficult period, and this quarter's low growth is a reflection of that reality," Amgen Chairman and Chief Executive Officer Kevin Sharer says.
The primary challenge for Amgen in the quarter has been questions about the safety of its erythropoeisis-stimulating agent (ESA) Aranesp, its best selling drug. In March, the company expanded the drug's label to warn patients of serious, sometimes deadly side effects when used to treat anemia in cancer patients. A Food & Drug Administration panel subsequently recommended limiting the drug's use to patients whose hemoglobin levels fall below a certain threshold and, even then, only for shorter periods of time.
As a result, Aranesp sales fell 10% in the second quarter to $949 million, driven by a 19% drop in demand in the U.S.
The rest of the year is unlikely to get any better for Aranesp, points out Friedman, Billings & Ramsey analyst James F. Reddoch. Just days after Amgen released its second-quarter results, the Centers for Medicare & Medicaid Services issued a final decision on how it would reimburse for Aranesp and other ESAs used in a cancer setting. The agency determined it would no longer cover ESA treatment for patients with a hemoglobin level of 10 g per deciliter or higher.
"Because of the tight range now established, we think Aranesp use in oncology will continue to fall sequentially for the next two quarters," Reddoch says. He has lowered his third- and fourth-quarter U.S. sales estimates to $445 million and $385 million, respectively, down from $551 million and $420 million.
Sales of Epogen, another ESA marketed by Amgen, increased 2% in the quarter to $624 million. Because the drug is used to treat anemia in patients with kidney failure or who are on dialysis, rather than in a cancer setting, Epogen is unaffected by the reimbursement changes.
The drug, however, may soon face new competition from Roche and its drug Cera. Amgen will go to court next month to try to keep the drug out of the U.S., claiming that it violates several Epogen patents.
Amgen's non-ESA portfolio posted relatively steady results. Sales of the arthritis drug Enbrel were up 14% to $823 million, while Neupogen and Neulasta, which treat a white-blood-cell disorder in cancer patients, brought in combined sales of $1.0 billion, a 4% rise. Sales of the cancer drug Vectibix, impacted by new unfavorable clinical-trial results, declined to $45 million, compared with $51 million in the first quarter of 2007.
Genentech grappled with a lack of new drivers of growth. Although the company enjoyed a 36.6% jump in revenues to $3.0 billion, with earnings up 38.5% to $834.0 million, it is heavily reliant on older products.
Avastin, its antibody approved to treat both colon and lung cancers, continues to be a critical growth engine for Genentech. The drug brought in $564 million, up 33% over the prior year. But analysts are concerned that Avastin may have already reached its peak penetration in lung cancer patients, a population considered an important growth opportunity for the drug.
OTHER PRODUCTS in the company's oncology portfolio had a less impressive quarter. Sales of lung cancer drug Tarceva, approved in 2004, fell 1% to $102 million. Sales of the breast cancer drug Herceptin were up a modest 3% to $329 million.
Lucentis, a treatment for wet, age-related macular degeneration, brought in $209 million in the quarter, about even with sales in the first quarter of the year.
Biogen Idec, though also burdened with older products, had a relatively healthy quarter. Earnings were up 21.8% to $240 million, based on a 17.1% increase in revenues to $773 million.
Much of the improvement came from growth in its multiple sclerosis franchise. Sales of Avonex were up 8% to $462 million. The newer product Tysabri brought in $72 million, a significant improvement over first-quarter sales of $48 million. Biogen took home $48 million of that total, while Elan Pharmaceuticals, its codevelopment partner for the drug, banked the remainder.
Tysabri could soon get another boost, as an FDA advisory committee last month recommended approving the drug to treat Crohn's disease.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter