Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Breaking Up Is Hard To Do
Researchers find a new way to split dinitrogen
by Sarah Everts
August 27, 2007
| A version of this story appeared in
Volume 85, Issue 35
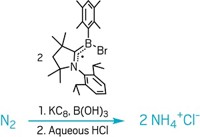
THE NARCISSISTIC EMBRACE of two nitrogen atoms makes N2 one of the most unreactive diatomic molecules in the natural world. Now, French researchers have found a new way to split up this stalwart, self-centered couple, using a single tantalum atom as the activator (Science 2007, 317, 1056).
Chemists Jean-Marie Basset, Alessandra Quadrelli, and Mostafa Taoufik at the University of Lyon and their colleagues in Lyon and at the University of Montpellier report the stoichiometric cleavage and subsequent hydrogenation of N2 on silica-supported tantalum hydride, using N2 and H2 gas as starting materials.
"Much of the activation to make ammonia in the past has relied on a multiple-metal-atom surface," Basset says. "We can activate a dinitrogen molecule with just one metal atom."
This new mechanism for splitting triply bonded N2 is "a significant and exciting advance," comments Paul J. Chirik, a chemist at Cornell University who works on dinitrogen functionalization. "Findings such as these inspire chemists to search for new ways to utilize atmospheric nitrogen as a reagent for the synthesis of commodity and fine chemicals."
Chemists figured out how to catalyze N2 hydrogenation just before World War I, using a cornucopia of transition metals such as Fe and Ru. The now-famous Haber-Bosch process provided a reliable source of munitions and fertilizer, impacting agriculture worldwide as well as other industrial processes requiring usable nitrogen. But this way of producing ammonia eats up swaths of energy—as much as 1% of the world's total energy consumption by some estimates.
For decades, researchers have been searching for new ways to catalyze the triple-bond split of N2 with a homogeneous catalyst that uses a modicum of energy, with some success. In 2003, for example, Richard R. Schrock and his colleagues at MIT reported what, at the time, was considered the best mechanistically defined system to catalytically reduce N2 to ammonia, although the efficiency was not optimal (C&EN, July 7, 2003, page 6). The catalyst is a molybdenum complex with hulking hexaisopropylterphenyl ligands.
The new work is mechanistically distinct from the Schrock system in that the new N–H bonds are formed directly from H2 gas, not from a sequential addition of protons from acids and then of electrons from reducing agents, Chirik notes. "A potentially new pathway to cleave and hydrogenate dinitrogen is enticing," he adds.
Basset's team is now working on making the reaction catalytic instead of stoichiometric. In addition, they are thinking about the variety of other reactions that might be catalyzed with their tantalum hydride center.
For example, the tantalum center is the same one that his team previously used to catalytically activate C–H and C–C bonds for alkane metathesis. As such, the researchers are hoping to eventually produce amines, "ideally from nitrogen gas and paraffins, which are inexpensive," Basset says. Although this is a long way off, the group is making some progress with the hydroamination of alkynes, Quadrelli notes.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter