Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Tetracycline synthesis improves
August 27, 2007
| A version of this story appeared in
Volume 85, Issue 35
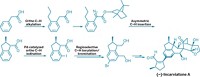
In 2005, Andrew G. Myers and colleagues at Harvard University reported an enantioselective semisynthetic route to tetracyclines that yielded an unprecedented series of structurally diverse analogs of this broad-spectrum class of antibiotics. In an ongoing effort to develop a fully synthetic route to tetracyclines, Myers and graduate student Jason D. Brubaker now report a completely different pathway to a key precursor in the synthesis (Org. Lett., DOI: 10.1021/ol071377d). The precursor (shown) contains the A and B rings of the four-ring tetracycline molecule. Important steps in the new route, which proceeds in nine steps with 21% overall yield on a multigram scale, include enantioselective addition of divinylzinc to a heterocyclic starting material and an endo-selective intramolecular Diels-Alder cycloaddition. "We believe that this innovation will fuel the discovery of large numbers of new candidate antibiotics and may provide the basis for novel tetracyclines to be synthesized on a scale sufficient for their clinical development," they write.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter