Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Clarifying HIV Entry Process
New details of virus's embrace with cell surface
by Stu Borman
October 1, 2007
| A version of this story appeared in
Volume 85, Issue 40

HIV infects immune cells by first using its surface glycoprotein to engage with a cell surface receptor and a coreceptor in a three-way embrace.
Long-standing efforts to structurally analyze this interaction have failed. But senior investigator Carole A. Bewley and structural biologist Peter D. Kwong of the National Institutes of Health and coworkers, including Son N. Lam and Chih-chin Huang in Bewley's and Kwong's groups, respectively, have now succeeded.
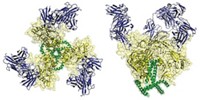
They've obtained the first structural view of the interaction between HIV envelope glycoprotein gp120, receptor CD4, and coreceptor CCR5 (Science 2007, 317, 1930). The work could help scientists design inhibitors that block HIV's entry in a new way.
In 1998, a team including Kwong and structural biologist Wayne A. Hendrickson of Howard Hughes Medical Institute and Columbia University obtained the structure of gp120 bound to CD4. That work "was a pretty heroic effort on its own," Hendrickson says. But the capability to analyze a complex also including CCR5 has been even more elusive.
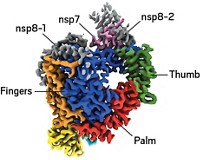
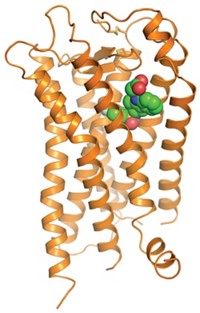
CCR5 is a G-protein coupled receptor (GPCR). These membrane proteins are virtually impossible to analyze structurally, so Kwong, Bewley, and coworkers used an N-terminal CCR5 fragment that includes two sulfated tyrosines known to interact with gp120. The researchers used crystallography, NMR spectroscopy, and molecular docking to obtain the structure of the three-part complex. They then confirmed the structure with specialized NMR techniques and mutagenesis and by comparing it with a related structure of a complex of gp120, CD4, and a CCR5-like antibody called 412d.
The study reveals that one of CCR5's sulfated tyrosines binds in a deep pocket on gp120 and is extensively hydrogen-bonded, whereas the other is more superficially bound and shares only two H-bonds with gp120. A known interaction between gp120 and an interior CCR5 loop is missing from the new structure; including that feature is a goal for future studies.
Because GPCRs are so difficult to analyze structurally, "getting a portion of CCR5 that would interact with gp120 in a significant way is a major achievement," Hendrickson says. He says the new study could aid the discovery of agents that block gp120 binding to CCR5. CCR5 is a promising drug target "because individuals missing CCR5 tend to be resistant to HIV and are perfectly healthy," he says.
Chemistry professor Amos B. Smith III at the University of Pennsylvania says the study "provides a new snapshot of the molecular machinery required for attachment, fusion, and insertion of HIV RNA into human T-cells. As such, it sets the stage for developing promising new therapies for the treatment of the AIDS pandemic, as current treatments based on reverse transcriptase and protease inhibitors yield increasingly to drug resistance."
Irwin Chaiken, professor of biochemistry and molecular biology at Drexel University College of Medicine, points out that although the approved drug Selzentry binds and blocks CCR5, there are currently no approved HIV entry inhibitors that bind gp120, so the study's identification of a new gp120 target site could be critical. "Even the 412d antibody itself could be a lead for small-molecule drug design," he says. "Also, the new target site might be useful as a reporter for inhibitors that bind at different gp120 sites and act synergistically with CCR5-gp120 inhibitors to block HIV entry."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter