Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Metal Binding Arrests Umbrella Inversion
October 15, 2007
| A version of this story appeared in
Volume 85, Issue 42
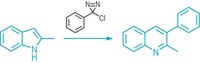
Metal coordination chemistry has been used to bestow controllable switching properties on an oscillating nitrogen ring, a result that could find application in nanoscale devices (Chem. Commun., DOI: 10.1039/b712447c). Nitrogen's lone pair of electrons rapidly fluctuates in many nitrogen-containing compounds, interconverting a compound between two enantiomeric forms in a process called pyramidal or umbrella inversion. Now, U.K.-based researchers led by Michael Shipman of the University of Warwick and James H. R. Tucker of the University of Birmingham demonstrate that inversion of the nitrogen atom in the three-membered aziridine ring of a pyridine-aziridine compound can be arrested by coordinating the two nitrogen atoms with a palladium salt as shown. Adding a bisphosphine ligand to scavenge the palladium frees the aziridine nitrogen's lone pair so the ring can resume its flip-flop motion. The researchers used NMR spectroscopy to quantify the umbrella inversion and to observe multiple cycles of on-off switching.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter