Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Macrocyclic Peptide Displays High Affinity for Key Protein
October 22, 2007
| A version of this story appeared in
Volume 85, Issue 43
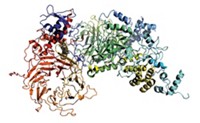
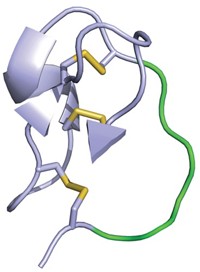
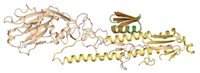
Researchers report that an iterative selection technique allowed them to identify a macrocyclic peptide with extraordinarily high affinity for a key signaling protein, suggesting that the technique, in their words, "provides a general route to design novel, low-molecular-weight, high-affinity ligands that target protein surfaces," (ACS Chem. Biol. 2007, 2, 625). Richard W. Roberts of the University of Southern California and coworkers carried out the study using mRNA display, an in vitro evolution method for identifying peptides that recognize specific targets. They generated about 1 trillion macrocyclic peptides containing a nonnatural amino acid and screened the molecules for binding to Gαi1, a signaling protein with potential relevance to several diseases. The selected macrocyclic peptide binds Gαi1 with 2-nM affinity—one of the strongest binding interactions ever described for peptides that recognize protein surfaces, Roberts says. Macrocyclic peptides containing nonnatural amino acids tend to be more stable and are predicted to enter cells more easily than linear peptides, making them promising drug candidates.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter