Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Membrane Protein Analyzed
New techniques could help make receptor structures more accessible
by Stu Borman
October 29, 2007
| A version of this story appeared in
Volume 85, Issue 44

SCIENTISTS WOULD LOVE to learn more about G-protein-coupled receptors (GPCRs), one of the most important families of proteins in the human body and a common target of drugs. But GPCRs have been almost impossible to analyze structurally. Only one crystal structure, that of the light-sensitive protein rhodopsin, has been obtained.
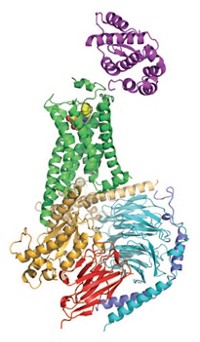
It's taken the better part of a decade, but now the second GPCR structure, that of the human β2 adrenergic receptor (β2AR), is finally making its debut—twice, via two different approaches. Researchers will now be trying to adapt techniques used to solve the structures for analyzing other GPCR structures much more quickly than has been possible before. The ability to analyze GPCRs more easily has major implications for drug discovery and could lead to a better understanding of receptor biology.
GPCRs are snakelike proteins that thread their way through cell membranes seven times, with their N-termini on the outside, C-termini on the inside, and flexible connecting loops on both sides. The receptors respond to hormones and neurotransmitters by transmitting cellular signals that affect myriad biological functions. And they are targets of many drugs that perturb those functions, including agents for diseases ranging from ulcers and migraines to cancer and stroke.
It was therefore frustrating that no GPCR structure was known until 2000, when a collaborative group analyzed rhodopsin (Science 2000, 289, 739; C&EN, Aug. 7, 2000, page 11). GPCR crystal structures are difficult to obtain because the receptors' extended, floppy conformations make them extraordinarily hard to crystallize. Take them out of their membrane homes, and they fall apart.
Now two teams have sidestepped that problem for β2AR by stabilizing it in different ways.
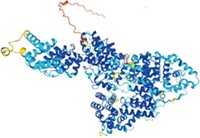
Biochemist Brian K. Kobilka of Stanford University School of Medicine and coworkers stabilized the protein by binding an antibody fragment to the GPCR's third inside loop (Nature, DOI: 10.1038/nature06325; Nat. Methods, DOI: 10.1038/nmeth1112).
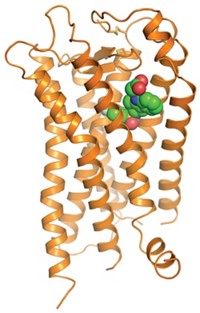
The other team, consisting of Kobilka, structural biologist Raymond C. Stevens of Scripps Research Institute, and coworkers, enhanced the receptor's stability by engineering a fusion protein in which the GPCR's third inside loop is replaced by the enzyme T4 lysozyme (Science, DOI: 10.1126/science.1150577 and 10.1126/science.1150609). In addition, this team used a crystallization technology called the lipidic cubic phase method to obtain the enzyme-stabilized structure.
The antibody-bound structure has 3.4-Å resolution. The enzyme-stabilized structure crystallized better, permitting analysis at a more detailed 2.4-Å resolution. In addition, extracellular loops are visible only in the enzyme-stabilized structure, easing analysis of critical ligand-receptor interactions. In other respects, however, the structures overlap closely and validate each other.
Rhodopsin, analyzed in 2000, is more suitable for crystallographic analysis than most GPCRs because it's available in large amounts (from cow retinas). Its unusual stability also makes it easier to crystallize. The new approaches that have now succeeded with the more difficult β2AR protein could help break the logjam of membrane proteins awaiting crystallographic structural analysis.
Professor of biological structure Ronald E. Stenkamp of the University of Washington, Seattle, comments that the new structures "are certainly exciting and long-sought results. There will be a lot of people reading these papers looking for new ideas to apply to their particular GPCRs."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter