Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Catalysis In Scandinavia
Regional history and present-day activity tie Nordic countries to catalytic chemistry
by Mitch Jacoby
November 5, 2007
| A version of this story appeared in
Volume 85, Issue 45

BROAD SCIENTIFIC CONCEPTS don't usually materialize suddenly without precedent. They tend to evolve slowly from a collection of ideas and observations, but sometimes a single event hastens the process. That's what happened 170 years ago in Northern Europe, when Jöns Jacob Berzelius coined the term "catalysis" and described the "catalytic power" of substances apparently not consumed in chemical reactions that "awaken affinities by their mere presence."
As author of the Royal Swedish Academy of Sciences' annual chemistry reports, Berzelius—the eminent chemist who discovered cerium, selenium, silicon, and thorium—introduced the concept of catalysis to explain decomposition of hydrogen peroxide by metals, conversion of ethanol to acetic acid by platinum, and other observations reported by seminal thinkers of his day. More than a century and a half later, some scientists argue that Berzelius' writings, which eventually reached leading European scientific institutions, helped spur development of the field of catalysis, especially in Scandinavia, which today boasts numerous centers of catalysis research.
"It's my impression that the work of Berzelius had, and still has, an impact on catalysis research in the Nordic countries," says Magnus Skoglundh, a professor of chemical engineering at Chalmers University of Technology, Göteborg, Sweden. Skoglundh, who serves as director of Chalmers' Competence Center for Catalysis, notes that catalysis research today in Sweden, Norway, Finland, and Denmark still benefits in some ways from studies carried out in that region in the 1800s, but he adds that the biggest impact on catalysis research comes from industry.
A leading source of that kind of influence in the region is Danish catalyst manufacturer Haldor Topsøe, which, as Skoglundh points out, is a company that for decades has promoted excellent catalysis research in Denmark and other Nordic countries. Other examples of industry-driven catalysis research come from local automobile and truck manufacturers, such as Volvo and Saab. The needs of those companies and others affect research directions and priorities, for example in automotive emissions cleanup technology, which is one of the Chalmers center's focus areas.
"Catalysis is certainly strong in Nordic countries, and people in this area who work in that field know about its Scandinavian roots," says Dmitry Murzin, a professor of industrial chemistry at Åbo Akademi University, Turku, Finland. But tracing everything back to Berzelius is a bit of a stretch, he adds.
Whether the connection between catalysis in Northern Europe today and science history in that region is direct or indirect is open to discussion. What's clear, however, is that catalysis is a hot topic in that part of the world.

One way to gauge the level of activity in the field is to look at the region's professional societies. Denmark, Finland, Sweden, and Norway each have national catalysis societies that are involved in a variety of activities, such as organizing local symposia, seminars, and other science-based events.
The national organizations also promote participation in international meetings and help spread the word about their country's programs in catalysis. As a case in point, the Swedish Catalysis Society's website includes a detailed list that describes research activities and provides links and contact information for 23 groups in Sweden that work in catalysis research and development.
The four countries also participate jointly in the Nordic Catalysis Society, which aims to bring together catalysis enthusiasts throughout the area by organizing symposia and summer schools in catalysis and through other events that promote educational and professional scientific advancement.
As an example of activity on an even grander scale, the Scandinavian organizations collectively hosted the eighth international meeting of the European Federation of Catalysis Societies, Europacat VIII, at the end of August in Turku. Held in Scandinavia for the first time, the meeting drew a record-breaking 1,500 participants from 56 countries to the southwest Finnish city (known in Swedish as Åbo), according to Tapio Salmi, head of Åbo Akademi's Laboratory of Industrial Chemistry. The conference, which was organized by Salmi, Murzin, and Henrik Topsøe, a manager at Haldor Topsøe, covered a significantly broader range of catalysis topics than previous Europacat meetings and featured more than 1,500 technical presentations, many of which showcased work being conducted at Scandinavia's top research centers.

Some of those centers are based in Denmark's leading universities, including Technical University of Denmark (abbreviated DTU in Danish) and Aarhus University. At DTU, located just north of Copenhagen in Lyngby, the team headed by physics professor Jens K. Nørskov develops and applies computational methods to probe properties of catalytic solids and other materials. Traditionally, theoreticians have used a material's structure and composition as a computational starting point from which to calculate fundamental properties, such as activation barriers and binding energies, and then compared the theoretical results with experimental measurements or used the calculated results to help interpret such measurements.
"But now the big question is, 'Can we go the other way?' " Nørskov asks. "If we want to design a material—a catalyst, for example—to exhibit a specific set of properties, can we calculate the atomic-scale structure and chemical composition needed to make such a material?"
THEORETICAL METHODS may not be able to provide the entire body of information needed to meet that goal, Nørskov acknowledges. "But if we can learn to use theory to give us hints to narrow down the possibilities, that would be an enormous help." Aiming for that objective, Nørskov and coworkers at DTU's Center for Atomic-Scale Materials Design have developed quantum-mechanics-based high-throughput-screening methods to computationally evaluate large numbers of materials in search of promising candidates predicted to be endowed with a customized set of desirable properties. Ideally, that type of investigation would identify materials that can be tested experimentally.
In one study along those lines, postdoc Jeff Greeley, Nørskov, fellow physics professor Ib Chorkendorff, and their coworkers screened computationally more than 700 two-component transition-metal alloys in the form of thin surface films in search of a promising electrocatalyst for the hydrogen evolution reaction (2H+ + 2e- → H2). With due consideration to several factors including catalyst activity and stability in an electrochemical environment, the team predicted that a BiPt alloy would perform as well or better than pure platinum, the archetypical catalyst for that reaction. Guided by their theoretical result, the team prepared the catalyst, tested it experimentally, and found that indeed, BiPt outperforms pure platinum (Nat. Mater. 2006, 5, 909).
In related work, N??rskov, DTU assistant professor Thomas Bligaard, and their coworkers applied similar methods to find a suitable catalyst for the methanation reaction (CO + 3H2 → CH4 + H2O), which is used, for example, in ammonia synthesis to remove trace amounts of CO from the hydrogen feed gas. In addition to evaluating a large number of would-be catalysts on the basis of physical and chemical properties, the group also considered cost. They predicted that Ni3Fe and NiFe should perform nearly as well as far more expensive catalysts based on cobalt or ruthenium. They confirmed the predictions experimentally (J. Catal. 2006, 239, 501).

Elsewhere at DTU, the research group of chemistry professor Claus H. Christensen is devoted to advancing sustainable chemical processes. "Our basic plan is to use catalysis as a tool to transform abundant and renewable bioresources into fuels, base chemicals, and fine chemicals," he says.
In one recent study, Christensen and colleagues investigated the propensity of various nickel- and ruthenium-based catalysts to convert ethanol, a key biomass derivative, into hydrogen. The group found that of all catalysts studied, the ruthenium system was the most effective. In addition, they observed that adding small amounts of potassium to a nickel catalyst delayed the harmful buildup of a carbon layer on that material's surfaces, thereby extending the catalyst lifetime (Green Chem. 2007, 9, 1016).
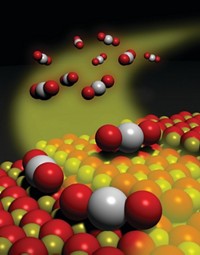
Understanding the surface properties of catalysts and other materials is a major undertaking of many research groups at the technical university, including that of Chorkendorff. His team's approach is based on state-of-the-art surface analysis methods. Using those kinds of tools, Chorkendorff, associate professor Jane H. Nielsen, and their coworkers discovered earlier this year that a low-cost sulfide, MoS2, is able to catalyze production of hydrogen from water, a reaction typically associated with expensive noble metals. Furthermore, the group deduced that the reaction occurs at catalyst sites found along the edges of flat single-layered MoS2 nanoparticles (C&EN, July 9, page 7).
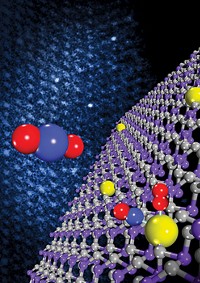
A more familiar use for molybdenum sulfide materials is hydrodesulfurization. In that process, sulfur contaminants in hydrocarbons—often in diesel fuel—are reacted with hydrogen in the presence of Co-Mo-S or Ni-Mo-S catalysts to produce volatile H2S, which is removed from the feed material. As legislation in the U.S., Europe, and elsewhere calls for ever-lower levels of sulfur in transportation fuels, scientists are trying to understand the atomic-scale processes that drive hydrodesulfurization. That type of work has been studied for years by the group of physics professor Flemming Besenbacher at Aarhus University, often in collaboration with scientists at Haldor Topsøe and at DTU.
On the basis of atomic-resolution scanning tunneling microscopy (STM) studies, computational methods, and other techniques, the researchers have shown that nanosized particles of MoS2 often assume a triangular shape that varies with the presence of nickel and cobalt catalyst promoters (activating additives). In addition, the particles frequently exhibit a "brim" just inside their outer edge. That brim, which appears bright in STM images because of its electronic nature, plays an important role in the reactions mediated by hydrodesulfurization catalysts, such as the ones manufactured by Haldor Topsøe.
Another way to keep down sulfur levels in transportation fuels is to make the fuels synthetically. That approach is being followed in several countries, including Finland, where oil manufacturer Neste Oil, located in Espoo, has recently begun converting vegetable oils and animal fats to biodiesel. Juha Jakkula, a manager with Neste, explains that the company's high-quality sulfur-free biodiesel is made by gasifying wood-based raw materials, which are abundant in Finland, or animal fats to make synthesis gas (a mixture of CO and hydrogen). The gas is then converted catalytically into paraffins.
The areas of catalysis practiced in Finland are shaped by natural factors, says Outi I. Krause, vice rector and professor of industrial chemistry at Helsinki University of Technology. "We don't have many natural resources in Finland, mainly wood and ore, but no oil or gas." Accordingly, there's a concentrated effort in developing renewable processes and catalytic technologies that benefit the environment. For example, Krause's group has studied methods for using CO2 as a feedstock as well as procedures for improving the quality of biofuels. Similarly, Riitta Keiski, a professor in University of Oulu's department of process and environmental engineering, works on automotive exhaust cleanup and air quality issues associated with malodorous sulfur compounds common in Finland's large pulp and paper industry.

IN CONTRAST, Norway has large offshore reserves of oil and gas. "Our focus in catalysis is mainly on converting and upgrading natural resources," says Erling Rytter, a specialist in oil refining at Statoil, an oil and gas company in Norway. One type of upgrading process studied at a number of institutions in Norway is a catalyzed transformation through which methanol (obtained, for example, from natural gas) is converted to hydrocarbons or to gasoline.
At the University of Oslo, postdoc Stian Svelle recently investigated catalyst deactivation and alkene formation within the methanol-to-hydrocarbons process. In that study, which was conducted with chemistry professor Unni Olsbye and coworkers at Oslo and Haldor Tops??e, the reaction was catalyzed by zeolite H-ZSM-5, a standard methanol-to-hydrocarbons catalyst. Among other observations, the team found that compounds such as trimethylbenzene are present as reaction intermediates and play a key role in forming ethene and propene. Larger compounds, such as hexamethylbenzene, play no such role. In addition, the researchers concluded that in contrast to large-pore zeolites, deactivation in H-ZSM-5 is a result of a carbon layer buildup on the exterior of the zeolites' pores—not the interior (J. Catal. 2007, 249, 195).
The interior of the catalyst is precisely where Karl Hult's attention is drawn. The biochemistry professor at the Royal Institute of Technology in Stockholm has been studying the active site of Candida antarctica lipase B, which is buried deep inside the enzyme, for signs of promiscuity. The term refers to an enzyme's ability to catalyze novel yet "unnatural" reactions or otherwise react in ways considered out of character for the enzyme. Recently, Hult and coworkers discovered that whereas the enzyme has evolved to hydrolyze esters, it can be used to synthesize difunctional polyesters containing terminal thiols and acrylates (Macromol. Rapid Commun. 2006, 27, 1932).
Advertisement
More than 170 years after Berzelius tried to organize a disparate collection of chemistry observations into a new category called catalytic reactions, the field of catalysis is thriving. As public attention on environmental and energy-related problems continues to grow, interest in catalysis as a potential problem solver—especially in catalysis hot spots like the Nordic countries—is sure to keep growing too. Of course, no single global region can claim a monopoly on catalytic chemistry. Just the same, if Berzelius were around nowadays, he'd no doubt be proud to see that catalysis has blossomed in his native Scandinavia.
MORE ON THIS STORY
- Catalysis In Scandinavia
- Regional history and present-day activity tie Nordic countries to catalytic chemistry
- Education
- Industry-Academic Collaborations Seen As Win-Win Situation



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter