Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
DNA Reveals New Reactions
November 19, 2007
| A version of this story appeared in
Volume 85, Issue 47
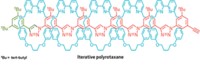
In 2004, David R. Liu and coworkers at Harvard University developed a DNA-based system for discovering new chemical reactions, but the conditions required for DNA base-pairing restricted its utility. Now, they have eliminated the need for base-pairing in their second-generation platform, which works in organic solvents and at high temperatures, conditions that are hostile to base-pairing (J. Am. Chem. Soc., DOI: 10.1021/ja074155j). The new setup uses a redesigned DNA architecture in which the sequence of a single DNA strand serves as readable bar code for the identity of two reactants, A and B (shown); the first system encoded each reactant on separate strands. Hundreds of different pairs of reactants are covalently attached to encoding strands, but in each case, one reactant is connected by a biotin-bearing disulfide linker. The system enables the scientists to efficiently discriminate between reactant pairs that form a covalent bond (red) under a given set of conditions and those that don't. The team uncovered what they hope is the first of many new reactions-a mild coupling between indoles and some alkenes.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter