Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
Risks From Foreign Drug Imports
Witnesses at House hearing express outrage over lack of FDA inspections
by Bette Hileman
November 26, 2007
| A version of this story appeared in
Volume 85, Issue 48

IN 2000, Food & Drug Administration officials told Congress that the agency inspected only a very small fraction of the facilities of the foreign firms that export finished pharmaceuticals or active ingredients into the U.S. Since then the situation has gotten much worse.
This month, witnesses at a hearing convened by the House Energy & Commerce Subcommittee on Oversight & Investigations disclosed a myriad of problems involving FDA's ability to ensure that imported drugs are safe. They testified that FDA can't even fully tabulate the foreign drug-producing firms that are shipping their products into the U.S. And witnesses also pointed out that even as the number of Chinese and Indian drugmakers exporting drugs to the U.S. has been ballooning, the percentage of facilities that FDA inspects is way down from what it was seven years ago.
The Nov. 1 hearing revealed an Alice-in-Wonderland situation regarding foreign manufacturers. It seems that FDA maintains two radically inconsistent databases listing foreign makers of finished drugs and active ingredients. One includes about 3,000 foreign establishments that were registered to market drugs or bulk active ingredients in the U.S. in fiscal 2007. However, the other database lists about 6,800 foreign firms that actually shipped drugs into the U.S. that year.
This ambiguous situation is muddied more by the fact that these lists were created using two different information technology systems and cannot be reconciled electronically, according to a Government Accountability Office (GAO) report presented at the hearing. Furthermore, FDA cannot verify the information in these databases, the report says, yet the agency relies on them when prioritizing its present list of 3,249 firms for inspection. "FDA's data are in shambles," Rep. Bart Stupak (D-Mich.), chair of the Oversight & Investigations Subcommittee, commented at the hearing.
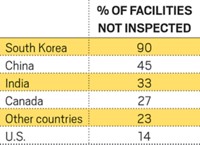
In fiscal 2007, of the thousands of foreign firms exporting pharmaceuticals into the U.S., 295 were inspected, Marcia G. Crosse, GAO's director of health care, told the subcommittee. In China, the country with the largest number of drug exporters, the agency inspected only 13 out of the at least 714 plants that send drugs to the U.S.
Crosse pointed out that the U.S. imports more pharmaceuticals than ever before-80% of the active pharmaceutical ingredients (APIs) and 40% of the finished drugs sold in the country. The value of such imports almost doubled between 2000 and 2005. "The U.S. increasingly relies on drugs from other countries, especially China and India," she said.
Unlike U.S. pharmaceutical manufacturers, which FDA must inspect once every two years, foreign firms that export drugs into the U.S. are not subject to a statutory inspection schedule. But many groups, including GAO, have recommended that the foreign firms be inspected with equal or greater frequency, Crosse said.
Moreover, she observed, FDA faces considerable handicaps when it inspects foreign firms. Unlike domestic plants, which are subject to unannounced inspections, foreign plants receive several weeks' advance notice of FDA's intent to visit. Because FDA does not generally have its own translators, inspectors must rely on English-speaking officials from foreign firms or governments during these visits. Also, when it appears that an inspection will require more time than has been scheduled, FDA does not have the flexibility to extend the tour. Usually, multiple inspections are arranged in the same country, and FDA personnel move on to the next facility on a fixed schedule. In contrast, when the agency discovers questionable practices in U.S. plants, it can extend the examination from a few days to two to three weeks.
IF THE LOW inspection rate of foreign firms continues, it will take FDA 55 years just to clear its backlog, John D. Dingell, (D-Mich.) House Energy & Commerce Committee chairman, said at the hearing. "The bottom line is that FDA has no clue what the condition is of most foreign drug-manufacturing facilities that export into the U.S. market," he added.
Carl R. Nielsen, former director of FDA's Division of Import Operations & Policy, testified that the agency applies the same techniques it uses to inspect food at U.S. borders to inspect foreign drug imports, but that these techniques are not suitable for drugs. "The current system is held together by baling wire and is incapable of determining or verifying the safety and efficacy of most imported drug products. It was broken in 1999, and it is even more so now," Nielsen said.
"Pharmaceuticals come in through about 250 ports of entry, and there is on average less than one person per port to inspect the shipments," he continued. The industry trend has been to move manufacturing of finished drugs and APIs offshore, but FDA's processes are entrenched in overseeing the domestic industry while largely ignoring foreign facilities, Nielsen said.
Potential dangers from foreign over-the-counter (OTC) drugs are even greater than those from prescription drugs, Nielsen said. Foreign OTC drug facilities are inspected once every 50 years on average, he noted, while prescription drug producers are examined about once a decade under the best-case scenario. He also noted that there is no requirement for the OTC industry to submit an application that describes its manufacturing processes, including its source of active ingredients.
Moreover, Nielsen said, testing of finished products alone is "inadequate to ensure a batch of product is safe and effective," stressing that inspection of the physical manufacturing plant, the processes, and the raw materials also is essential. Lack of foreign inspections also creates "an unfair competitive advantage for the foreign industry," he added.
"The current FDA organization is not designed and funded to adequately oversee foreign firms, to effectively manage and administer the related programs, and to ensure the delivery of safe and effective imported drug products into the U.S.," Nielsen concluded. "We have been sitting here talking about the same problems for the past 10 years. I will do everything I can to avoid coming back again in 10 years."
These problems are not unique to the U.S., said Guy Villax, the former chairman of the Pharmaceuticals Business Committee of the European Fine Chemicals Group. Like the U.S. system, the European Union drug regulatory system is "out of date" and "broken," he said. Villax is CEO of Hovione, a European supplier of APIs to the pharmaceutical industry.
DURING MOST of the 20th century, Villax said, medicines were manufactured primarily in Europe and the U.S. by large multinational firms and in compliance with recognized Good Manufacturing Practices. Now, "about 80% of the active pharmaceutical ingredients used to make medicines found in EU pharmacies comes from abroad, and not everyone is playing by the rules," he said. A large and increasing proportion of these ingredients originate in Asian countries, up from almost none 20 years ago, he said. "The vast majority of medicines are no longer produced by the large multinationals."
A multitude of companies that do not make their own APIs nonetheless are manufacturing the majority of medicines, most of which are generics and OTC drugs, Villax continued. In this battle for markets, "the least scrupulous operators, often middlemen, who can supply APIs at the lowest prices, are usually the winners," he said. "This is putting the safety of our citizens at risk.
"Oddly, the EU inspects API plants based on proximity, not risk," he said. In the past few years, according to Villax, the EU has found pharmaceutical production problems only in Asia. But European authorities inspect only 30 to 50 plants in Asia each year, he said, while they examine a much greater number in Europe. Several international bodies, such as the European Parliament and the World Health Organization (WHO), have recently recognized that "more inspections are key to preventing noncompliant APIs from reaching the market," he said.
William K. Hubbard, a former associate commissioner at FDA, also told the House panel that foreign drug products present a greater potential risk than domestically produced pharmaceuticals do. In foreign countries, he testified, "drugs are often purchased from suppliers who have little or no oversight by regulatory bodies." This means that key elements of safe drug production-such as quality testing, expiration dating, and labeling controls-are ignored and that "producers of substandard and counterfeit drugs have relatively easy access to the marketplace." China and India, which have weak drug regulatory systems, are the two largest foreign suppliers of drugs and drug ingredients to the U.S., and FDA inspectors have at times found horrendous conditions in their factories, Hubbard said.
These conditions have serious health implications. According to WHO, more than half of the drug supply in parts of Africa and Asia is counterfeit, and China is the principal world supplier of those fake drugs, Hubbard said. In China alone, an estimated 200,000 to 300,000 people die each year because of counterfeit drugs, he said. And increasing amounts of these dangerous products are reaching Europe. In just the past two years, seizures of fake drugs in the EU rose from 500,000 to almost 3 million tablets, he noted.
IDENTIFYING COUNTERFEIT drugs at the U.S. border is not an easy task, added Hubbard. "FDA's inspection rate for imported drugs when they arrive at a U.S. port is about 1%," and the chances of an imported drug ingredient being tested at entry is even lower, he said. Almost 2 million drug shipments, from small packages to containers with several metric tons of bulk APIs, cross the U.S. borders each year. But FDA labs tested only 340 shipments last year, he said.
Some stakeholders suggest "we should rely upon agreements negotiated with foreign countries" to ensure the safety of imported drugs, Hubbard said. "But I believe that developing countries are incapable of effectively implementing such agreements" and that "such a course of action is a prescription for frustration."

Another formula for failure when it comes to finding problems with imported products is FDA's reliance on border examinations, label review, and testing of finished products, testified attorney Benjamin L. England, of the Washington, D.C., law firm Jones Walker. Instead, FDA should rely on an assessment and understanding about the conditions of manufacture within the foreign facility, he told the committee. The results of testing shipments at the border "are anecdotal at best," he said. "FDA's foreign drug inspection program, its import program, and its information technology system were broken eight years ago and they remain broken today," he warned, echoing Hubbard's assessment.
England recommended steps to improve the safety of drug imports. One of them is that significant resources should be allocated to reengineering FDA's information technology systems. He also recommended that FDA establish an internal organization that reports directly to the commissioner about steps taken to improve the safety of drug imports. The organization should plan all import activities, inspect foreign drugmakers, and oversee border operations. It should be funded directly from the commissioner's office rather than receiving its support from the various FDA product centers, he said.
BOLSTERING the observations of the hearing witnesses and the data from the GAO's report, several congressional staff members from the Oversight and Investigations Subcommittee accompanied FDA personnel on inspections of four pharmaceutical companies in China and five in India. During these site visits in August, they talked with plant and government officials. The resulting report was entered as part of the official hearing record.
A few of the drug firms visited by the congressional team were in urban hubs, but most were in remote, rural areas far from where Chinese and Indian government inspectors are based, the report said. One Chinese facility in a mountainous region could be accessed only by helicopter because the primary road to the plant had been washed out by recent monsoons and had not yet been repaired.
The team chose to go on this particular inspection trip not only because China and India are major producers of APIs and finished drugs for the U.S. market, but also because their production is expected to expand greatly in the near future. This will add significantly to FDA's inspection responsibilities, the report noted.
The congressional investigators recommended that permanent FDA offices be established in India and China to facilitate the inspection process. The offices should help coordinate entry and movement of FDA inspectors within the countries and improve collaboration between the U.S., India, and China by helping train local regulatory inspectors. Government officials in India say they support the idea of a permanent FDA presence there, the report noted, but China has not yet expressed an opinion about allowing agency offices within its borders.
Advertisement
Amid all of this criticism, FDA contends it is working to address the issues raised by hearing witnesses. FDA Commissioner Andrew C. von Eschenbach testified that upgrading the agency's information technology systems is one of his top priorities. "We expect these improvements will help address the challenges we face in the area of foreign inspections," he said. One of the difficulties with tracking foreign firms that sell drugs to the U.S. is that many that are on the list of registered exporters have never sent any products to the U.S. or have stopped exporting without notifying U.S. authorities, he noted.
To help ensure the safety of imported drugs, von Eschenbach said, FDA has established a variety of cooperative relationships with foreign regulators, ranging from collaborations under free-trade agreements to agency-to-agency memorandums of understanding. In addition, he explained, "FDA exchanges inspectional information with many foreign partners. We are communicating with some of them on a daily, and sometimes hourly, basis."
FDA also is working on setting up permanent offices in foreign countries to help with inspections and to "build capacity," von Eschenbach said. "No one is satisfied with the situation as it exists today."
Also to promote and enhance the safety of all imported products, President George W. Bush convened the Interagency Working Group on Import Safety in July, von Eschenbach said. It was tasked with reviewing procedures, regulations, and practices that ensure imported drugs, food, and other consumer products are safe, he said.
The working group already is having an impact. On Nov. 6, the Bush Administration proposed a large expansion of the authority of FDA and the Consumer Product Safety Commission (CPSC) to inspect and certify drug, food, and other imports as a result of the group's recommendations. The plan proposes "steps to replace the current 'snapshot' approach to import safety, in which inspections are made at the border," with a prevention-focused system that "targets critical points in the import life cycle where risk is greatest."
The plan would involve stationing inspectors in foreign countries to examine drugs, food, and other products before they are exported to the U.S. It also recommends giving FDA authority to require foreign drug manufacturers in certain high-risk regions to seek certification showing that products meet U.S. safety standards. The names of certified producers and importers could be made public, although these details are still being worked out. Under the plan, importers of goods that violate U.S. laws would be given stiffer penalties than are currently in place.
Implementing the plan would require budget increases at FDA and CPSC. Legislation would also be required to give the agencies more power over imports. It is unclear, however, which parts of the plan could be executed with new regulations and budget increases and which parts will require new legislation.
CONGRESS IS not waiting for FDA to act. It is already considering legislation that takes a different approach to improving the safety of drug and food imports. In September, Dingell introduced a bill, H.R. 3610, that he says "would give FDA adequate resources to restructure and fund the foreign drug inspection program."
The bill would create and impose user fees on importers of food and drugs. Funds generated by the fees would be devoted to hiring additional inspectors at the U.S. border and abroad and to increase testing of imports at FDA labs. The bill also would require country-of-origin labeling of drugs and food. "The agency is using an antiquated regulatory system from the last century, when the global economy was very different," Dingell said.






Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter