Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Iron-Catalyzed C-N Cross-Couplings
December 3, 2007
| A version of this story appeared in
Volume 85, Issue 49
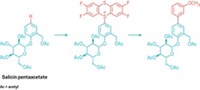
A promising iron-catalyzed reaction to make N-aryl pyrazoles and other aryl-substituted nitrogen compounds has been developed by Arkaitz Correa and Carsten Bolm at RWTH Aachen University, in Germany (Angew. Chem. Int. Ed. 2007, 46, 8862). The synthetic method offers a potentially less expensive and environmentally friendlier alternative to palladium- and copper-catalyzed C–N coupling reactions, the researchers say. The products of these reactions contain important structural motifs found in natural products and bioactive synthetic compounds and are essential intermediates in preparing pharmaceuticals and other chemicals. After exploring several iron salt precursors, the researchers settled on FeCl3. Under optimized conditions, FeCl3 paired with an ethylenediamine chelating ligand and a base in toluene solvent was used to convert aryl halides and pyrazole, indole, or other nitrogen-containing substrates into N-aryl products in moderate to high yields (one example shown). "The research has established a useful starting point for investigating future applications of iron-catalyzed N-arylation reactions," the scientists write.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter