Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Rolling Out The Oil
Chemistry and microstructure together repel organic liquids
by Carmen Drahl
December 10, 2007
| A version of this story appeared in
Volume 85, Issue 50
INSPIRED BY LOTUS LEAVES, scientists at MIT and the Air Force Research Laboratory have devised a route to super oil-repellent surfaces (Science 2007, 318, 1618). Materials that repel organic liquids could be used to clean up hazardous waste or to oil proof aircraft parts.


Courtesy of Anish Tuteja/Wonjae Choi
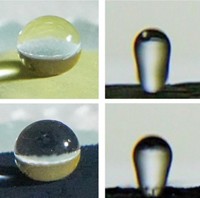
Scanning electron microscope image showing oil-repellent microfibers.
Lotus leaves naturally repel water and have inspired many synthetic water-repellent surfaces. These surfaces work like wax on a car, inducing water droplets to bead up on contact and roll away. But organic liquids spread out and wet these surfaces, because compared with water, they have lower surface tension—the attractive force that clusters molecules into droplets.
A team led by two MIT professors, mechanical engineer Gareth H. McKinley and chemical engineer Robert E. Cohen, circumvented the surface tension problem by using specially designed material structures to make surfaces oil-repellent.
According to Cohen, lotus leaves possess small structures that let water droplets ball up instead of spread out. The team reasoned that these features—bumps and nubs that curve underneath themselves—might be adapted to make oil-repellent surfaces.
To make curvy textures suitable for repelling organic liquids, the team took pointers from lotus leaves but altered both their surface chemistry and microscopic geometry. The right surface chemistry came from a polymer blended with an ultrahydrophobic material developed by researchers at Edwards Air Force Base, in California (C&EN, April 10, 2006, page 71). Using a process called electrospinning, the team created a tissue-thin microfiber material from the polymer blend that contained air pockets spaced and angled just right for cushioning oils at the surface.
Courtesy of Anish Tuteja/Wonjae Choi
An oil-repellent microfiber cloak applied to a lotus leaf lets droplets of hexadecane colored with a red dye bead up.
"Electrospinning can be scaled up to coat surfaces of various shapes and sizes," Cohen says, making it practical for oil-proofing applications. For example, the team gently wrapped microfibers around lotus leaves, creating coated surfaces that repel octane and hexadecane, as well as water.
The team also etched nanopillars and troughs into silicon surfaces with lithographic techniques developed in other labs. This helped the team understand the relationship between geometry and liquid repellence, which led to an electrospun surface that can separate water and octane, a component in some aircraft fuels. Such a surface could find use in fuel-spill cleanup. The result also "provides a practical path to nonwetting surfaces for advanced separations technologies," says Edward T. Samulski, professor of chemistry at the University of North Carolina, Chapel Hill.
The surfaces tend to lose their oil-repellent powers over time, and that could hinder some applications, according to A. Levent Demirel, associate professor of chemistry at Koç University, in Istanbul, Turkey. The work, however, is "a clear demonstration of the effect of concave topographic features" on liquid-repelling properties, he adds.
The team hopes that materials such as protective coatings for airplane parts vulnerable to fuel leaks will arise from their work. "It's the combination of chemistry on the nanoscale and fiber structure on the micrometer scale that gives rise to our materials' oil-repelling nature," McKinley says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter