Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Novel Embrace Of Protein And Metal
Unique interaction may give copper an easy out
by Celia Henry Arnaud
January 7, 2008
| A version of this story appeared in
Volume 86, Issue 1
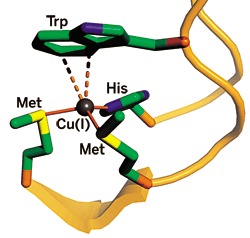
The bacterial copper-trafficking protein CusF recognizes Cu(I) through covalent bonds with methionine (Met) and histidine (His) and electrostatic cation-π interactions with tryptophan (Trp).
A COPPER-TRAFFICKING PROTEIN has been found to have a unique type of metal-binding mechanism that may ease copper release.
A team led by chemistry professor Thomas V. O'Halloran of Northwestern University reports that the bacterial copper-trafficking protein CusF binds copper via a strong cation-π interaction between Cu(I) and a key tryptophan (Nat. Chem. Bio., DOI: 10.1038/nchembio.2007.57). Proteins usually bind metals through covalent interactions.
CusF binds Cu(I) in a three-coordinate structure with two methionines and a histidine. Copper also interacts with the indole ring of a nearby tryptophan that stacks on top of it. The team used resonance Raman and X-ray spectroscopy to confirm that the Cu-tryptophan interaction is an electrostatic cation-π type. The Ag(I)-bound form of the binding site's structure, though not this interaction, was independently reported last year by a team led by biologist Megan M. McEvoy of the University of Arizona (Protein Sci. 2007, 16, 2287).
When the researchers replace the tryptophan with a methionine, the complex forms a fourth covalent bond and holds Cu more tightly. The lower affinity afforded by the tryptophan cation-π interaction may allow the copper-trafficking protein to release its metal cargo more easily, according to O'Halloran.
"What is surprising in the X-ray structures reported by O'Halloran and McEvoy is this intriguing tryptophan, which is very unusual for a metalloprotein binding pocket," says Katherine J. Franz, a chemistry professor at Duke University who studies metal ion coordination in biological systems.
O'Halloran's team is trying to answer some remaining questions, such as the role of the tryptophan in metal recognition. The tryptophan may protect copper from other ligands, O'Halloran says. He doesn't yet know how CusF releases the copper.
"It will be interesting to see if other proteins harbor copper-tryptophan cation-π interactions waiting to be uncovered," Franz says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter